Extracellular-Regulated Kinases: Signaling From Ras to ERK Substrates to Control Biological Outcomes
- PMID: 29551131
- PMCID: PMC6007982
- DOI: 10.1016/bs.acr.2018.02.004
Extracellular-Regulated Kinases: Signaling From Ras to ERK Substrates to Control Biological Outcomes
Abstract
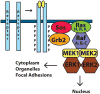
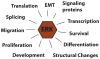
The extracellular-regulated kinases ERK1 and ERK2 are evolutionarily conserved, ubiquitous serine-threonine kinases that are involved in regulating cellular signaling in both normal and pathological conditions. Their expression is critical for development and their hyperactivation is a major factor in cancer development and progression. Since their discovery as one of the major signaling mediators activated by mitogens and Ras mutation, we have learned much about their regulation, including their activation, binding partners and substrates. In this review I will discuss some of what has been discovered about the members of the Ras to ERK pathway, including regulation of their activation by growth factors and cell adhesion pathways. Looking downstream of ERK activation I will also highlight some of the many ERK substrates that have been discovered, including those involved in feedback regulation, cell migration and cell cycle progression through the control of transcription, pre-mRNA splicing and protein synthesis.
Keywords: Adhesion; ERK; Feedback; MEK; Proliferation; RAS; Raf; Signaling; Substrates.
© 2018 Elsevier Inc. All rights reserved.
Figures

References
-
- Ahn NG, Krebs EG. Evidence for an epidermal growth factor-stimulated protein kinase cascade in Swiss 3T3 cells. Activation of serine peptide kinase activity by myelin basic protein kinases in vitro. J Biol Chem. 1990;265(20):11495–11501. - PubMed
-
- Ahn NG, Seger R, Bratlien RL, Diltz CD, Tonks NK, Krebs EG. Multiple components in an epidermal growth factor-stimulated protein kinase cascade. In vitro activation of a myelin basic protein/microtubule-associated protein 2 kinase. J Biol Chem. 1991;266(7):4220–4227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

