Ribosome Levels Selectively Regulate Translation and Lineage Commitment in Human Hematopoiesis
- PMID: 29551269
- PMCID: PMC5866246
- DOI: 10.1016/j.cell.2018.02.036
Ribosome Levels Selectively Regulate Translation and Lineage Commitment in Human Hematopoiesis
Abstract
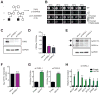
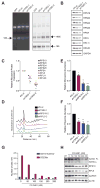
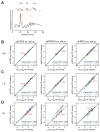
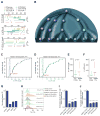
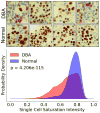
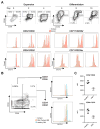
Blood cell formation is classically thought to occur through a hierarchical differentiation process, although recent studies have shown that lineage commitment may occur earlier in hematopoietic stem and progenitor cells (HSPCs). The relevance to human blood diseases and the underlying regulation of these refined models remain poorly understood. By studying a genetic blood disorder, Diamond-Blackfan anemia (DBA), where the majority of mutations affect ribosomal proteins and the erythroid lineage is selectively perturbed, we are able to gain mechanistic insight into how lineage commitment is programmed normally and disrupted in disease. We show that in DBA, the pool of available ribosomes is limited, while ribosome composition remains constant. Surprisingly, this global reduction in ribosome levels more profoundly alters translation of a select subset of transcripts. We show how the reduced translation of select transcripts in HSPCs can impair erythroid lineage commitment, illuminating a regulatory role for ribosome levels in cellular differentiation.
Keywords: Diamond-Blackfan anemia; GATA1; erythropoiesis; genetics; hematopoiesis; lineage commitment; ribosome; translation.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
There are no competing interests to disclose.
Figures

References
-
- Allam R, Chennupati V, Veiga DFT, Maslowski KM, Tardivel A, Quadroni M, Duchosal M, MacDonald HR, Fasel N, Angelillo-Scherrer A, et al. An Unexpected Role for Ribonuclease Inhibitor (RNH1) in Erythropoiesis. Blood. 2014;124:244–244.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

