Pharmacokinetic principles in the inner ear: Influence of drug properties on intratympanic applications
- PMID: 29551306
- PMCID: PMC6133771
- DOI: 10.1016/j.heares.2018.03.002
Pharmacokinetic principles in the inner ear: Influence of drug properties on intratympanic applications
Abstract
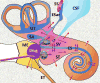
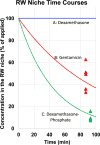
Local drug delivery to the ear has gained wide clinical acceptance, with the choice of drug and application protocol in humans largely empirically-derived. Here, we review the pharmacokinetics underlying local therapy of the ear using the drugs commonly used in clinical practice as examples. Based on molecular properties and perilymph measurements interpreted through computer simulations we now better understand the principles underlying entry and distribution of these and other drugs in the ear. From our analysis, we have determined that dexamethasone-phosphate, a pro-drug widely-used clinically, has molecular and pharmacokinetic properties that make it ill-suited for use as a local therapy for hearing disorders. This polar form of dexamethasone, used as a more soluble agent in intravenous preparations, passes less readily through lipid membranes, such as those of the epithelia restricting entry at the round window membrane and stapes. Once within the inner ear, dexamethasone-phosphate is cleaved to the active form, dexamethasone, which is less polar, passes more readily through lipid membranes of the blood-perilymph barrier and is rapidly eliminated from perilymph without distributing to apical cochlear regions. Dexamethasone-phosphate therefore provides only a brief exposure of the basal regions of the cochlea to active drug. Other steroids, such as triamcinolone-acetonide, exhibit pharmacokinetic properties more appropriate to the ear and merit more detailed consideration.
Keywords: Drug delivery; Inner ear; Intracochlear; Intralabyrinthine; Oval window; Perilymph; Round window membrane.
Copyright © 2018 Elsevier B.V. All rights reserved.
Figures






References
-
- Alexander TH, Harris JP, Nguyen QT, Vorasubin N. Dose Effect of Intratympanic Dexamethasone for Idiopathic Sudden Sensorineural Hearing Loss: 24 mg/mL Is Superior to 10 mg/mL. Otol Neurotol. 2015;36:1321–1327. - PubMed
-
- Ayoob AM, Borenstein JT. The role of intracochlear drug delivery devices in the management of inner ear disease. Expert Opin Drug Deliv. 2015;12:465–479. - PubMed
-
- Bird PA, Begg EJ, Zhang M, Keast AT, Murray DP, Balkany TJ. Intratympanic versus intravenous delivery of methylprednisolone to cochlear perilymph. Otol Neurotol. 2007;28:1124–1130. - PubMed
-
- Briggs R, O’leary S, Birman C, et al. A first time in human investigation of a combination device delivering a targeted drug therapy to cochlear implant recipients. 14th International Conference on Cochlear Implants; Toronto. 2016.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

