Expression and regulation of alarmin cytokine IL-1α in human retinal pigment epithelial cells
- PMID: 29551335
- PMCID: PMC6076434
- DOI: 10.1016/j.exer.2018.03.015
Expression and regulation of alarmin cytokine IL-1α in human retinal pigment epithelial cells
Abstract
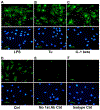
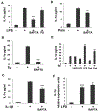
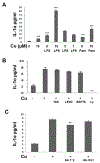
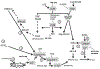
Human retinal pigment epithelial (hRPE) cells play important immune-regulatory roles in a variety of retinal pathologic processes, including the production of inflammatory cytokines that are essential mediators of the innate immune response within the ocular microenvironment. The pro-inflammatory "alarmin" cytokine IL-1α has been implicated in both infectious and non-infectious retinal diseases, but its regulation in the retina is poorly understood. The purpose of this study was to elucidate the expression and regulation of IL-1α within hRPE cells. To do this, IL-1α mRNA and protein in hRPE cells was assessed by RT-PCR, qPCR, ELISA, Western blot, and immunofluorescence following treatment with a variety of stimuli and inhibitors. ER stress, LPS, IL-1β, and TLR2 activation all significantly increased intracellular IL-1α protein. Increasing intracellular calcium synergized both LPS- and Pam3CSK4-induced IL-1α protein production. Accordingly, blocking calcium signaling and calpain activity strongly suppressed IL-1α protein expression. Significant but more moderate inhibition occurred following blockage of TLR4, caspase-4, or caspase-1. Neutralizing antibodies to IL-1β and TLR2 partially eliminated LPS- and TLR2 ligand Pam3CSK4-stimulated IL-1α protein production. IFN-β induced caspase-4 expression and activation, and also potentiated LPS-induced IL-1α expression, but IFN-β alone had no effect on IL-1α protein production. Interestingly, all inhibitors targeting the PI3K/Akt pathway, with the exception of Ly294002, strongly increased IL-1α protein expression. This study improves understanding of the complex mechanisms regulating IL-1α protein expression in hRPE cells by demonstrating that TLR4 and TLR2 stimulation and exposure to IL-1β, ER stress and intracellular calcium all induce hRPE cells to produce intracellular IL-1α, which is negatively regulated by the PI3K/Akt pathway. Additionally, the non-canonical inflammasome pathway was shown to be involved in LPS-induced hRPE IL-1α expression through caspase-4 signaling.
Keywords: Caspase-4; IL-1α; Interleukin-1α; Non-canonical inflammasome; RPE; Retinal pigment epithelium; TLR2; TLR4.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures




References
-
- Aksoy E, Taboubi S, Torres D, Delbauve S, Hachani A, Whitehead MA, Pearce WP, Berenjeno IM, Nock G, Filloux A, Beyaert R, Flamand V, Vanhaesebroeck B, 2012. The p110delta isoform of the kinase PI(3)K controls the subcellular compartmentalization of TLR4 signaling and protects from endotoxic shock. Nat Immunol 13, 1045–1054. - PMC - PubMed
-
- Bandyopadhaya A, Bhowmick S, Chaudhuri K, 2009. Activation of proinflammatory response in human intestinal epithelial cells following Vibrio cholerae infection through PI3K/Akt pathway. Can J Microbiol 55, 1310–1318. - PubMed
-
- Bian ZM, Elner SG, Yoshida A, Elner VM, 2003. Human RPE-monocyte co-culture induces chemokine gene expression through activation of MAPK and NIK cascade. Experimental eye research 76, 573–583. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

