Bayesian adaptive bandit-based designs using the Gittins index for multi-armed trials with normally distributed endpoints
- PMID: 29551849
- PMCID: PMC5856359
- DOI: 10.1080/02664763.2017.1342780
Bayesian adaptive bandit-based designs using the Gittins index for multi-armed trials with normally distributed endpoints
Abstract
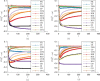
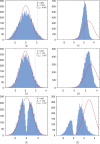
Adaptive designs for multi-armed clinical trials have become increasingly popular recently because of their potential to shorten development times and to increase patient response. However, developing response-adaptive designs that offer patient-benefit while ensuring the resulting trial provides a statistically rigorous and unbiased comparison of the different treatments included is highly challenging. In this paper, the theory of Multi-Armed Bandit Problems is used to define near optimal adaptive designs in the context of a clinical trial with a normally distributed endpoint with known variance. We report the operating characteristics (type I error, power, bias) and patient-benefit of these approaches and alternative designs using simulation studies based on an ongoing trial. These results are then compared to those recently published in the context of Bernoulli endpoints. Many limitations and advantages are similar in both cases but there are also important differences, specially with respect to type I error control. This paper proposes a simulation-based testing procedure to correct for the observed type I error inflation that bandit-based and adaptive rules can induce.
Keywords: Gittins index; Multi-armed bandit; normally distributed endpoint; patient allocation; response adaptive procedures; sequential sampling.
Conflict of interest statement
Disclosure statement No potential conflict of interest was reported by the authors.
Figures






References
-
- do Amaral J.F.P., Aspects of optimal sequential resource allocation, D.Phil. thesis, University of Oxford, 1985.
-
- Atkinson A.C. and Biswas A., Randomised Response-Adaptive Designs in Clinical Trials, CRC Press, Boca Raton, FL, 2014.
-
- Auer P., Cesa-Bianchi N., and Fischer P., Finite-time analysis of the multiarmed bandit problem , Mach. Learn. 47 (2002), pp. 235–256. doi: 10.1023/A:1013689704352 - DOI
-
- Bather J., Randomized allocation of treatments in sequential trials , Adv. Appl. Probab. 12 (1980), pp. 174–182. doi: 10.1017/S0001867800033449 - DOI
-
- Berry D.A., [Investigating therapies of potentially great benefit: ECMO]: comment: ethics and ECMO , Stat. Sci. 4 (1989), pp. 306–310. doi: 10.1214/ss/1177012385 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
