Role of σ,π-Digold(I) Alkyne Complexes in Reactions of Enynes
- PMID: 29551852
- PMCID: PMC5850095
- DOI: 10.1021/acs.organomet.7b00668
Role of σ,π-Digold(I) Alkyne Complexes in Reactions of Enynes
Abstract
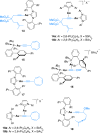
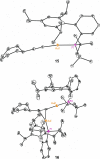
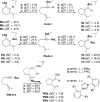
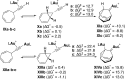
Gold(I) acetylide and σ,π-digold(I) alkyne complexes derived from one prototypical 1,6-enyne and from 7-ethynyl-1,3,5-cycloheptatriene have been prepared and structurally characterized. Their possible role in gold(I)-catalyzed cycloisomerizations has been studied by experiment and by DFT calculations. Gold(I) acetylides are totally unproductive complexes in the absence of Brønsted acids. Similarly, no cyclizations were observed by heating σ,π-digold(I) alkyne digold(I) at least up to 130 °C. Theoretical studies provide a rationale for the much lower reactivity of digold species in reactions of enynes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
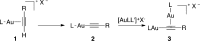


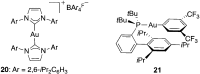

References
-
- Braun I.; Asiri A. M.; Hashmi A. S. K. ACS Catal. 2013, 3, 1902–1907. 10.1021/cs400437s. - DOI
-
- Seidel G.; Lehmann C. W.; Fürstner A. Angew. Chem., Int. Ed. 2010, 49, 8466–8470. 10.1002/anie.201003349. - DOI - PubMed
- Hooper T. N.; Green M.; Russell C. A. Chem. Commun. 2010, 46, 2313–2315. 10.1039/b923900f. - DOI - PubMed
- Brown T. J.; Widenhoefer R. A. Organometallics 2011, 30, 6003–6009. 10.1021/om200840g. - DOI
- Brown T. J.; Widenhoefer R. A. J. Organomet. Chem. 2011, 696, 1216–1220. 10.1016/j.jorganchem.2010.09.055. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
