Induction and Subversion of Human Protective Immunity: Contrasting Influenza and Respiratory Syncytial Virus
- PMID: 29552008
- PMCID: PMC5840263
- DOI: 10.3389/fimmu.2018.00323
Induction and Subversion of Human Protective Immunity: Contrasting Influenza and Respiratory Syncytial Virus
Abstract
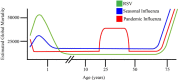
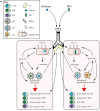
Respiratory syncytial virus (RSV) and influenza are among the most important causes of severe respiratory disease worldwide. Despite the clinical need, barriers to developing reliably effective vaccines against these viruses have remained firmly in place for decades. Overcoming these hurdles requires better understanding of human immunity and the strategies by which these pathogens evade it. Although superficially similar, the virology and host response to RSV and influenza are strikingly distinct. Influenza induces robust strain-specific immunity following natural infection, although protection by current vaccines is short-lived. In contrast, even strain-specific protection is incomplete after RSV and there are currently no licensed RSV vaccines. Although animal models have been critical for developing a fundamental understanding of antiviral immunity, extrapolating to human disease has been problematic. It is only with recent translational advances (such as controlled human infection models and high-dimensional technologies) that the mechanisms responsible for differences in protection against RSV compared to influenza have begun to be elucidated in the human context. Influenza infection elicits high-affinity IgA in the respiratory tract and virus-specific IgG, which correlates with protection. Long-lived influenza-specific T cells have also been shown to ameliorate disease. This robust immunity promotes rapid emergence of antigenic variants leading to immune escape. RSV differs markedly, as reinfection with similar strains occurs despite natural infection inducing high levels of antibody against conserved antigens. The immunomodulatory mechanisms of RSV are thus highly effective in inhibiting long-term protection, with disturbance of type I interferon signaling, antigen presentation and chemokine-induced inflammation possibly all contributing. These lead to widespread effects on adaptive immunity with impaired B cell memory and reduced T cell generation and functionality. Here, we discuss the differences in clinical outcome and immune response following influenza and RSV. Specifically, we focus on differences in their recognition by innate immunity; the strategies used by each virus to evade these early immune responses; and effects across the innate-adaptive interface that may prevent long-lived memory generation. Thus, by comparing these globally important pathogens, we highlight mechanisms by which optimal antiviral immunity may be better induced and discuss the potential for these insights to inform novel vaccines.
Keywords: B cell; CD4+ T cell; RIG-I like receptor; influenza; innate immunity; respiratory disease; respiratory syncytial virus; toll-like receptor.
Figures

References
-
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (2015) 385(9963):117–71. 10.1016/S0140-6736(14)61682-2 - DOI - PMC - PubMed
-
- Office for National Statistics. Deaths Registered in England and Wales (Series DR): 2015. London: Office for National Statistics; (2016). Available from: https://www.ons.gov.uk/releases/deathsregisteredinenglandandwalesseriesd...
-
- Pfuntner A, Wier LM, Stocks C. Most Frequent Conditions in U.S. Hospitals, 2011: HCUP Statistical Brief #162. Rockville, MD: Agency for Healthcare Research and Quality; (2013). - PubMed
-
- Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child (1986) 140(6):543–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

