In Utero Exposure to Exosomal and B-Cell Alloantigens Lessens Alloreactivity of Recipients' Lymphocytes Rather than Confers Allograft Tolerance
- PMID: 29552016
- PMCID: PMC5840197
- DOI: 10.3389/fimmu.2018.00418
In Utero Exposure to Exosomal and B-Cell Alloantigens Lessens Alloreactivity of Recipients' Lymphocytes Rather than Confers Allograft Tolerance
Abstract
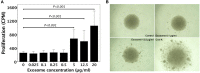
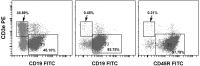
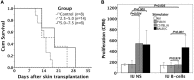
According to actively acquired tolerance, antigen exposure before full immune development in fetal or early neonatal life will cause tolerance to this specific antigen. In this study, we aimed to examine whether allogeneic tolerance could be elicited by in utero exposure to surface MHC antigens of allogenic cells or soluble form of MHC exosomes. Gestational day 14 FVB/N fetuses were subjected to intraperitoneal injection of allogeneic major histocompatibility complex (MHC) exosomes or highly enriched B-cells. Postnatally, the recipients were examined for the immune responses to donor alloantigens by lymphocyte proliferative reactions and skin transplantation. In utero exposure to allogeneic MHC exosomes abolished the alloreactivity of recipients' lymphocytes to the alloantigens, but could not confer skin allograft tolerance. In utero transplantation of highly enriched allogeneic B-cells generated low-level B-cell chimerism in the recipients. However, it only extended the survivals of skin allograft by a few days despite the lack of donor-specific alloreactivity of recipients' lymphocyte. Thus, an early in utero contact with exosomal or B-cell alloantigens did not lead to full skin tolerance but rather, at best, only to delayed skin rejection in the presence of microchimerism made by B-cell inocula. These results argued against the theory of actively acquired tolerance, and implicated that in utero exposure to marrow cells in previous studies was a unique model of allo-tolerance induction that involved the establishment of significant hematopoietic chimerism. Taken together with the discovery of in utero sensitization to ovalbumin in our previous studies, the immunological consequences of fetal exposure to foreign antigens might vary according to the type or nature of antigens introduced.
Keywords: B-cells; alloreactivity; exosome; in utero injection; major histocompatibility complex; tolerance induction.
Figures


Similar articles
-
Immunological Consequences of In Utero Exposure to Foreign Antigens.Front Immunol. 2021 Apr 15;12:638435. doi: 10.3389/fimmu.2021.638435. eCollection 2021. Front Immunol. 2021. PMID: 33936052 Free PMC article. Review.
-
Completely allogeneic spleen cells induced cytolytic neonatal tolerance to alloantigens, but failed to establish allo-helper interactions with host T cells.Immunology. 1996 Nov;89(3):413-8. doi: 10.1046/j.1365-2567.1996.d01-763.x. Immunology. 1996. PMID: 8958055 Free PMC article.
-
Allogeneic lymphocytes exerted graft-versus-host rather than tolerogenic effects on preimmune fetuses.J Surg Res. 2013 Jul;183(1):405-11. doi: 10.1016/j.jss.2012.12.015. Epub 2013 Jan 2. J Surg Res. 2013. PMID: 23295194
-
Differences in non-MHC alloantigens promote tissue rejection but fail to mediate allogeneic co-operation and autoimmunity in mice neonatally injected with semi-allogeneic F1 B cells.Immunology. 1994 Jun;82(2):287-93. Immunology. 1994. PMID: 7927500 Free PMC article.
-
Emerging role of exosomes in allorecognition and allograft rejection.Curr Opin Organ Transplant. 2018 Feb;23(1):22-27. doi: 10.1097/MOT.0000000000000489. Curr Opin Organ Transplant. 2018. PMID: 29189413 Free PMC article. Review.
Cited by
-
Clinical relevance of feto-maternal microchimerism in (hematopoietic stem cell) transplantation.Semin Immunopathol. 2024 Dec 7;47(1):4. doi: 10.1007/s00281-024-01028-3. Semin Immunopathol. 2024. PMID: 39644358 Free PMC article. Review.
-
Immunological Consequences of In Utero Exposure to Foreign Antigens.Front Immunol. 2021 Apr 15;12:638435. doi: 10.3389/fimmu.2021.638435. eCollection 2021. Front Immunol. 2021. PMID: 33936052 Free PMC article. Review.
-
Fetal exposure to oncoantigen elicited antigen-specific adaptive immunity against tumorigenesis.J Immunother Cancer. 2020 Jun;8(1):e000137. doi: 10.1136/jitc-2019-000137. J Immunother Cancer. 2020. PMID: 32561637 Free PMC article.
-
Extracellular vesicles and immune response during pregnancy: A balancing act.Immunol Rev. 2022 Jul;308(1):105-122. doi: 10.1111/imr.13074. Epub 2022 Feb 23. Immunol Rev. 2022. PMID: 35199366 Free PMC article. Review.
References
-
- Argyris BF. Acquired tolerance to skin homografts in mice. II. Role of donor cell population in inducing and maintaining tolerance. J Immunol (1964) 92:630–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

