Post-operative unadjuvanted therapeutic xenovaccination with chicken whole embryo vaccine suppresses distant micrometastases and prolongs survival in a murine Lewis lung carcinoma model
- PMID: 29552144
- PMCID: PMC5840525
- DOI: 10.3892/ol.2018.7950
Post-operative unadjuvanted therapeutic xenovaccination with chicken whole embryo vaccine suppresses distant micrometastases and prolongs survival in a murine Lewis lung carcinoma model
Abstract
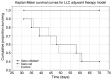
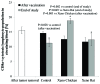
Immunotherapy in the form of anticancer vaccination relies on the mobilization of the patient's immune system against specific cancer antigens. Instead of focusing on an autologous cell lysate, which is not always available in clinical practice, the present study investigates vaccines utilizing xenogeneic foetal tissue that are rich in oncofoetal antigens. Lewis lung carcinoma (LLC)-challenged C57BL/6 mice were treated with either a xenogeneic vaccine made from chicken whole embryo, or a xenogeneic vaccine made from rat embryonic brain tissue, supplemented with a Bacillus subtilis protein fraction as an adjuvant. Median and overall survival, size of metastatic foci in lung tissue and levels of circulating CD8a+ T cells were evaluated and compared with untreated control mice. Following primary tumour removal, a course of three subcutaneous vaccinations with xenogeneic chicken embryo vaccine led to significant increase in overall survival rate (100% after 70 days of follow-up vs. 40% in untreated control mice), significant increase in circulating CD8a+ T cells (18.18 vs. 12.6% in untreated control mice), and a significant decrease in the area and incidence of metastasis foci. The xenogeneic rat brain tissue-based vaccine did not improve any of the investigated parameters, despite promising reports in other models. We hypothesize that the proper selection of antigen source (tissue) can constitute an effective immunotherapeutic product.
Keywords: Lewis lung carcinoma; cytotoxic lymphocytes; metastasis; mice; vaccination; xenogeneic.
Figures



Similar articles
-
Bacterial ghosts as adjuvants in syngeneic tumour cell lysate-based anticancer vaccination in a murine lung carcinoma model.Oncol Rep. 2017 Jan;37(1):171-178. doi: 10.3892/or.2016.5252. Epub 2016 Nov 16. Oncol Rep. 2017. PMID: 27878261
-
The anticancer efficiency of the xenogeneic vaccine and the indication for its use.Exp Oncol. 2014 Jun;36(2):79-84. Exp Oncol. 2014. PMID: 24980760
-
The effects of early postoperative immunization with xenogeneic embryo proteins on Lewis lung carcinoma model.Exp Oncol. 2018 Dec;40(4):275-281. Exp Oncol. 2018. PMID: 30593747
-
Immune modulations during chemoimmunotherapy & novel vaccine strategies--in metastatic melanoma and non small-cell lung cancer.Dan Med J. 2013 Dec;60(12):B4774. Dan Med J. 2013. PMID: 24355457 Review.
-
Xenogeneic therapeutic cancer vaccines as breakers of immune tolerance for clinical application: to use or not to use?Vaccine. 2014 Jul 7;32(32):4015-24. doi: 10.1016/j.vaccine.2014.05.006. Epub 2014 May 14. Vaccine. 2014. PMID: 24837511 Review.
References
-
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 [Internet] Lyon, France: 2013.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
