MicroRNA-125a-5p inhibits invasion and metastasis of gastric cancer cells by targeting BRMS1 expression
- PMID: 29552146
- PMCID: PMC5840665
- DOI: 10.3892/ol.2018.7983
MicroRNA-125a-5p inhibits invasion and metastasis of gastric cancer cells by targeting BRMS1 expression
Abstract
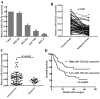
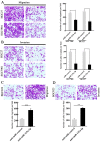
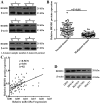
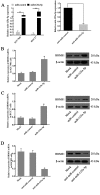
Accumulating studies have demonstrated microRNAs (miRNAs/miRs) have an important role in multiple processes of human malignant tumor development and progression. Decreased expression of miR-125a-5p has been observed in several types of cancer, including gastric cancer (GC). However, the mechanism and exact function of miR-125a-5p in GC have not been largely elucidated. In the present study, reverse transcription-quantitative polymerase chain reaction indicated that the expression of miR-125a-5p was downregulated in GC tissues and cell lines compared with matched normal tissues (P<0.01) and normal gastric mucosa cell lines (P<0.01), respectively. Moreover, clinical pathological characteristics and Kaplan-Meier analysis indicated that a low expression of miR-125a-5p was not only associated with lymph metastasis, peritoneal dissemination and advanced tumor-node metastasis stage but also affected the prognosis of GC patients. Compared with miR-control-transfected GC cells, markedly decreased migration and invasion was observed in GC cells that overexpress miR-125a-5p. By contrast, increased metastasis and invasion were observed in miR-125a-5p-knocked down cells compared with the control. Furthermore, luciferase reporter assays indicated that breast cancer metastasis suppressor 1 (BRMS1) was a direct target of miR-125a-5p. Notably, a positive correlation between the levels of BRMS1 and miR-125a-5p in GC tissues was observed, and BRMS1 expression was indicated to be regulated by miR-125a-5p in GC cells. In conclusion, miR-125a-5p may act as a tumor suppressor by targeting the metastasis-inhibitory gene, BRMS1. The data suggesting that BRMS1 is a potential target gene of miR-125a-5p, may provide novel insight into miRNA regulation of human gene expression, and a useful target for gene therapy of GC.
Keywords: Invasion and metastasis; breast cancer metastasis suppressor 1; gastric cancer; microRNA-125a-5p.
Figures

Similar articles
-
Epigenetics mechanisms mediate the miR-125a/BRMS1 axis to regulate invasion and metastasis in gastric cancer.Onco Targets Ther. 2019 Sep 12;12:7513-7525. doi: 10.2147/OTT.S210376. eCollection 2019. Onco Targets Ther. 2019. PMID: 31571904 Free PMC article.
-
Prognostic value of microRNA-125a/b family in patients with gastric cancer: a meta-analysis.Gastroenterol Hepatol Bed Bench. 2021 Fall;14(Suppl1):S1-S9. Gastroenterol Hepatol Bed Bench. 2021. PMID: 35154597 Free PMC article. Review.
-
Hsa-miR-125a-3p and hsa-miR-125a-5p are downregulated in non-small cell lung cancer and have inverse effects on invasion and migration of lung cancer cells.BMC Cancer. 2010 Jun 22;10:318. doi: 10.1186/1471-2407-10-318. BMC Cancer. 2010. PMID: 20569443 Free PMC article.
-
Low expression of miR-125a-5p is associated with poor prognosis in patients with gastric cancer.Oncol Lett. 2019 Aug;18(2):1483-1490. doi: 10.3892/ol.2019.10423. Epub 2019 May 31. Oncol Lett. 2019. PMID: 31423214 Free PMC article.
-
STAT3 Pathway in Gastric Cancer: Signaling, Therapeutic Targeting and Future Prospects.Biology (Basel). 2020 Jun 12;9(6):126. doi: 10.3390/biology9060126. Biology (Basel). 2020. PMID: 32545648 Free PMC article. Review.
Cited by
-
Epigenetics mechanisms mediate the miR-125a/BRMS1 axis to regulate invasion and metastasis in gastric cancer.Onco Targets Ther. 2019 Sep 12;12:7513-7525. doi: 10.2147/OTT.S210376. eCollection 2019. Onco Targets Ther. 2019. PMID: 31571904 Free PMC article.
-
Immunomodulatory Properties of Human Breast Milk: MicroRNA Contents and Potential Epigenetic Effects.Biomedicines. 2022 May 24;10(6):1219. doi: 10.3390/biomedicines10061219. Biomedicines. 2022. PMID: 35740242 Free PMC article. Review.
-
FOXS1 is regulated by GLI1 and miR-125a-5p and promotes cell proliferation and EMT in gastric cancer.Sci Rep. 2019 Mar 27;9(1):5281. doi: 10.1038/s41598-019-41717-w. Sci Rep. 2019. PMID: 30918291 Free PMC article.
-
miR-125a-5p post-transcriptionally suppresses GALNT7 to inhibit proliferation and invasion in cervical cancer cells via the EGFR/PI3K/AKT pathway.Cancer Cell Int. 2020 Apr 10;20:117. doi: 10.1186/s12935-020-01209-8. eCollection 2020. Cancer Cell Int. 2020. PMID: 32308562 Free PMC article.
-
Prognostic value of microRNA-125a/b family in patients with gastric cancer: a meta-analysis.Gastroenterol Hepatol Bed Bench. 2021 Fall;14(Suppl1):S1-S9. Gastroenterol Hepatol Bed Bench. 2021. PMID: 35154597 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
