Pancreatic adenocarcinoma, chronic pancreatitis, and MODY-8 diabetes: is bile salt-dependent lipase (or carboxyl ester lipase) at the crossroads of pancreatic pathologies?
- PMID: 29552330
- PMCID: PMC5844766
- DOI: 10.18632/oncotarget.23619
Pancreatic adenocarcinoma, chronic pancreatitis, and MODY-8 diabetes: is bile salt-dependent lipase (or carboxyl ester lipase) at the crossroads of pancreatic pathologies?
Abstract
Pancreatic adenocarcinomas and diabetes mellitus are responsible for the deaths of around two million people each year worldwide. Patients with chronic pancreatitis do not die directly of this disease, except where the pathology is hereditary. Much current literature supports the involvement of bile salt-dependent lipase (BSDL), also known as carboxyl ester lipase (CEL), in the pathophysiology of these pancreatic diseases. The purpose of this review is to shed light on connections between chronic pancreatitis, diabetes, and pancreatic adenocarcinomas by gaining an insight into BSDL and its variants. This enzyme is normally secreted by the exocrine pancreas, and is diverted within the intestinal lumen to participate in the hydrolysis of dietary lipids. However, BSDL is also expressed by other cells and tissues, where it participates in lipid homeostasis. Variants of BSDL resulting from germline and/or somatic mutations (nucleotide insertion/deletion or nonallelic homologous recombination) are expressed in the pancreas of patients with pancreatic pathologies such as chronic pancreatitis, MODY-8, and pancreatic adenocarcinomas. We discuss the possible link between the expression of BSDL variants and these dramatic pancreatic pathologies, putting forward the suggestion that BSDL and its variants are implicated in the cell lipid metabolism/reprogramming that leads to the dyslipidemia observed in chronic pancreatitis, MODY-8, and pancreatic adenocarcinomas. We also propose potential strategies for translation to therapeutic applications.
Keywords: bile salt-dependent lipase; carboxyl ester lipase; chronic pancreatitis; diabetes; pancreatic adenocarcinoma.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures
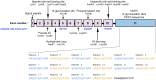
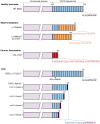


References
-
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. - PubMed
-
- Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH, Neoptolemos JP. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. - PubMed
-
- Pliarchopoulou K, Pectasides D. Pancreatic cancer: current and future treatment strategies. Cancer Treatment Review. 2009;35:431–436. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

