The Pharmacology of T Cell Therapies
- PMID: 29552577
- PMCID: PMC5852291
- DOI: 10.1016/j.omtm.2018.01.010
The Pharmacology of T Cell Therapies
Abstract
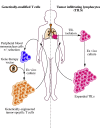
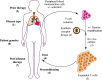
Adoptive cellular therapy using T cells with tumor specificity derived from either natural T cell receptors (TCRs) or an artificial chimeric antigen receptor (CAR) has reached late phase clinical testing, with two CAR T cell therapies achieving regulatory approval within the United States in 2017. The effective use of these therapies depends upon an understanding of their pharmacology, which is quite divergent from traditional small molecule or biologic drugs. We review the different types of T cell therapy under clinical development, the factors affecting cellular kinetics following infusion, and the relationship between these cellular kinetics and anti-cancer activity. We also discuss the toxicity associated with T cell therapies, with an emphasis on cytokine release syndrome and neurotoxicity, and the gaps in knowledge regarding these frequent and unique adverse effects.
Keywords: T cell; cellular therapy; chimeric antigen receptor; gene therapy; immunotherapy.
Figures
References
-
- Novartis AG. (2017). Kymriah package insert. https://www.fda.gov/downloads/UCM573941.pdf.
-
- Kite Pharma, Inc. (2017). Yescarta package insert. https://www.fda.gov/downloads/UCM581226.pdf.
-
- Horowitz M.M., Gale R.P., Sondel P.M., Goldman J.M., Kersey J., Kolb H.J., Rimm A.A., Ringdén O., Rozman C., Speck B. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. - PubMed
-
- Topalian S.L., Muul L.M., Solomon D., Rosenberg S.A. Expansion of human tumor infiltrating lymphocytes for use in immunotherapy trials. J. Immunol. Methods. 1987;102:127–141. - PubMed
-
- Dréno B., Nguyen J.M., Khammari A., Pandolfino M.C., Tessier M.H., Bercegeay S., Cassidanius A., Lemarre P., Billaudel S., Labarrière N. Randomized trial of adoptive transfer of melanoma tumor-infiltrating lymphocytes as adjuvant therapy for stage III melanoma. Cancer Immunol. Immunother. 2002;51:539–546. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

