Plastin 3 Promotes Motor Neuron Axonal Growth and Extends Survival in a Mouse Model of Spinal Muscular Atrophy
- PMID: 29552580
- PMCID: PMC5852384
- DOI: 10.1016/j.omtm.2018.01.007
Plastin 3 Promotes Motor Neuron Axonal Growth and Extends Survival in a Mouse Model of Spinal Muscular Atrophy
Abstract
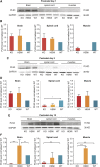
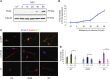
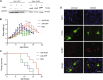
Spinal muscular atrophy (SMA) is a devastating childhood motor neuron disease. SMA is caused by mutations in the survival motor neuron gene (SMN1), leading to reduced levels of SMN protein in the CNS. The actin-binding protein plastin 3 (PLS3) has been reported as a modifier for SMA, making it a potential therapeutic target. Here, we show reduced levels of PLS3 protein in the brain and spinal cord of a mouse model of SMA. Our study also revealed that lentiviral-mediated PLS3 expression restored axonal length in cultured Smn-deficient motor neurons. Delivery of adeno-associated virus serotype 9 (AAV9) harboring Pls3 cDNA via cisterna magna in SMNΔ7 mice, a widely used animal model of SMA, led to high neuronal transduction efficiency. PLS3 treatment allowed a small but significant increase of lifespan by 42%. Although there was no improvement of phenotype, this study has demonstrated the potential use of Pls3 as a target for gene therapy, possibly in combination with other disease modifiers.
Keywords: SMA; gene therapy; plastin 3.
Figures

References
-
- Stratigopoulos G., Lanzano P., Deng L., Guo J., Kaufmann P., Darras B., Finkel R., Tawil R., McDermott M.P., Martens W. Association of plastin 3 expression with disease severity in spinal muscular atrophy only in postpubertal females. Arch. Neurol. 2010;67:1252–1256. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

