Hypoxia Impairs Mesenchymal Stromal Cell-Induced Macrophage M1 to M2 Transition
- PMID: 29552603
- PMCID: PMC5854485
- DOI: 10.1142/S2339547817500042
Hypoxia Impairs Mesenchymal Stromal Cell-Induced Macrophage M1 to M2 Transition
Abstract
The transition of macrophages from the pro-inflammatory M1 to the anti-inflammatory M2 phenotype is crucial for the progression of normal wound healing. Persistent M1 macrophages within the injury site may lead to an uncontrolled macrophage-mediated inflammatory response and ultimately a failure of the wound healing cascade, leading to chronic wounds. Mesenchymal stromal cells (MSCs) have been widely reported to promote M1 to M2 macrophage transition; however, it is unclear whether MSCs can drive this transition in the hypoxic environment typically observed in chronic wounds. Here we report on the effect of hypoxia (1% O2) on MSCs' ability to transition macrophages from the M1 to the M2 phenotype. While hypoxia had no effect on MSC secretion, it inhibited MSC-induced M1 to M2 macrophage transition, and suppressed macrophage expression and production of the anti-inflammatory mediator interleukin-10 (IL-10). These results suggest that hypoxic environments may impede the therapeutic effects of MSCs.
Figures
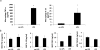
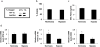
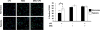
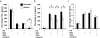
Similar articles
-
Age-Related Effects on MSC Immunomodulation, Macrophage Polarization, Apoptosis, and Bone Regeneration Correlate with IL-38 Expression.Int J Mol Sci. 2024 Mar 13;25(6):3252. doi: 10.3390/ijms25063252. Int J Mol Sci. 2024. PMID: 38542230 Free PMC article.
-
Effects of the fibrous topography-mediated macrophage phenotype transition on the recruitment of mesenchymal stem cells: An in vivo study.Biomaterials. 2017 Dec;149:77-87. doi: 10.1016/j.biomaterials.2017.10.007. Epub 2017 Oct 4. Biomaterials. 2017. PMID: 29017079
-
Pseudomonas aeruginosa infection alters the macrophage phenotype switching process during wound healing in diabetic mice.Cell Biol Int. 2018 Jul;42(7):877-889. doi: 10.1002/cbin.10955. Epub 2018 Mar 14. Cell Biol Int. 2018. PMID: 29512223
-
Extracardiac-Lodged Mesenchymal Stromal Cells Propel an Inflammatory Response Against Myocardial Infarction via Paracrine Effects.Cell Transplant. 2016;25(5):929-35. doi: 10.3727/096368915X689758. Epub 2015 Oct 22. Cell Transplant. 2016. PMID: 26498018 Review.
-
Bone regeneration in inflammation with aging and cell-based immunomodulatory therapy.Inflamm Regen. 2023 May 25;43(1):29. doi: 10.1186/s41232-023-00279-1. Inflamm Regen. 2023. PMID: 37231450 Free PMC article. Review.
Cited by
-
Macrophage modulation by polymerized hemoglobins: Potential as a wound-healing therapy.Technology (Singap World Sci). 2019 Sep-Dec;7(3n04):84-97. doi: 10.1142/s2339547819500055. Epub 2020 Jan 21. Technology (Singap World Sci). 2019. PMID: 38486857 Free PMC article.
-
The Immune-Centric Revolution in the Diabetic Foot: Monocytes and Lymphocytes Role in Wound Healing and Tissue Regeneration-A Narrative Review.J Clin Med. 2022 Feb 8;11(3):889. doi: 10.3390/jcm11030889. J Clin Med. 2022. PMID: 35160339 Free PMC article. Review.
-
Autologous cell therapy in diabetes‑associated critical limb ischemia: From basic studies to clinical outcomes (Review).Int J Mol Med. 2021 Sep;48(3):173. doi: 10.3892/ijmm.2021.5006. Epub 2021 Jul 19. Int J Mol Med. 2021. PMID: 34278463 Free PMC article. Review.
-
Advances and Challenges in Immune-Modulatory Biomaterials for Wound Healing Applications.Pharmaceutics. 2024 Jul 26;16(8):990. doi: 10.3390/pharmaceutics16080990. Pharmaceutics. 2024. PMID: 39204335 Free PMC article. Review.
-
The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes.Front Physiol. 2018 May 1;9:419. doi: 10.3389/fphys.2018.00419. eCollection 2018. Front Physiol. 2018. PMID: 29765329 Free PMC article. Review.
References
-
- Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–289. - PubMed
-
- Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383–396. - PubMed
-
- Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, Hainzl A, Schatz S, Qi Y, Schlecht A, Weiss JM, Wlaschek M, Sunderkotter C, Scharffetter-Kochanek K. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121:985–997. - PMC - PubMed
-
- Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
