Experimental Nonalcoholic Steatohepatitis and Liver Fibrosis Are Ameliorated by Pharmacologic Activation of Nrf2 (NF-E2 p45-Related Factor 2)
- PMID: 29552625
- PMCID: PMC5852394
- DOI: 10.1016/j.jcmgh.2017.11.016
Experimental Nonalcoholic Steatohepatitis and Liver Fibrosis Are Ameliorated by Pharmacologic Activation of Nrf2 (NF-E2 p45-Related Factor 2)
Abstract
Background & aims: Nonalcoholic steatohepatitis (NASH) is associated with oxidative stress. We surmised that pharmacologic activation of NF-E2 p45-related factor 2 (Nrf2) using the acetylenic tricyclic bis(cyano enone) TBE-31 would suppress NASH because Nrf2 is a transcriptional master regulator of intracellular redox homeostasis.
Methods: Nrf2+/+ and Nrf2-/- C57BL/6 mice were fed a high-fat plus fructose (HFFr) or regular chow diet for 16 weeks or 30 weeks, and then treated for the final 6 weeks, while still being fed the same HFFr or regular chow diets, with either TBE-31 or dimethyl sulfoxide vehicle control. Measures of whole-body glucose homeostasis, histologic assessment of liver, and biochemical and molecular measurements of steatosis, endoplasmic reticulum (ER) stress, inflammation, apoptosis, fibrosis, and oxidative stress were performed in livers from these animals.
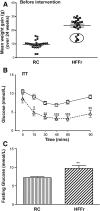
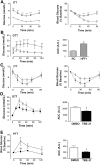
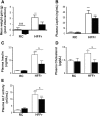
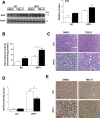
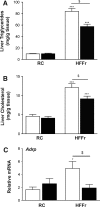
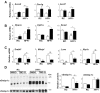
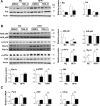
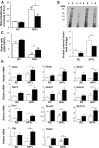
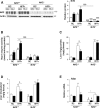
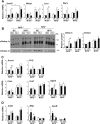
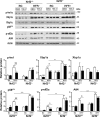
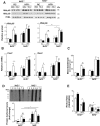
Results: TBE-31 treatment reversed insulin resistance in HFFr-fed wild-type mice, but not in HFFr-fed Nrf2-null mice. TBE-31 treatment of HFFr-fed wild-type mice substantially decreased liver steatosis and expression of lipid synthesis genes, while increasing hepatic expression of fatty acid oxidation and lipoprotein assembly genes. Also, TBE-31 treatment decreased ER stress, expression of inflammation genes, and markers of apoptosis, fibrosis, and oxidative stress in the livers of HFFr-fed wild-type mice. By comparison, TBE-31 did not decrease steatosis, ER stress, lipogenesis, inflammation, fibrosis, or oxidative stress in livers of HFFr-fed Nrf2-null mice.
Conclusions: Pharmacologic activation of Nrf2 in mice that had already been rendered obese and insulin resistant reversed insulin resistance, suppressed hepatic steatosis, and mitigated against NASH and liver fibrosis, effects that we principally attribute to inhibition of ER, inflammatory, and oxidative stress.
Keywords: ACACA, acetyl-CoA carboxylase alpha; ACLY, ATP citrate lyase; ACOT7, acetyl-CoA thioesterase 7; ACOX2, acetyl-CoA oxidase 2; ADRP, adipose differentiation-related protein; AP-1, activator protein 1; ATF4, activating transcription factor-4; ATF6, activating transcription factor-6; ApoB, apolipoprotein B; BCL-2, B-cell lymphoma; BIP, binding immunoglobulin protein; C/EBP, CCAAT/enhancer-binding protein; CAT, catalase; CD36, cluster of differentiation 36; CDDO, 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid; CES1G, carboxylesterase 1g; CHOP, C/EBP homologous protein; COL1A1, collagen, type I, alpha-1; COX2, cyclooxygenase-2; CPT1A, carnitine palmitoyltransferase 1a; ChREBP, carbohydrate-responsive element-binding protein; DGAT2, diacylglycerol acyltransferase-2; DMSO, dimethyl sulfoxide; ER, endoplasmic reticulum; FASN, fatty acid synthase; FXR, farnesoid X receptor; GCLC, glutamate-cysteine ligase catalytic; GCLM, glutamate-cysteine ligase modifier; GPX2, glutathione peroxidase-2; GSH, reduced glutathione; GSSG, oxidized glutathione; GSTA4, glutathione S-transferase Alpha-4; GSTM1, glutathione S-transferase Mu-1; GTT, glucose tolerance test; H&E, hematoxylin and eosin; HF, high-fat; HF30Fr, high-fat diet with 30% fructose in drinking water; HF55Fr, high-fat diet with 55% fructose in drinking water; HFFr, high-fat diet with fructose in drinking water; HMOX1, heme oxygenase-1; IKK, IκB kinase; IRE1α, inositol requiring kinase-1α; ITT, insulin tolerance test; IκB, inhibitor of NF-κB; JNK1, c-Jun N-terminal kinase 1; Keap1, Kelch-like ECH-associated protein-1; LXRα, liver X receptor α; MCD, methionine- and choline-deficient; MCP-1, monocyte chemotactic protein-1; MGPAT, mitochondrial glycerol-3-phosphate acetyltransferase; MPO, myeloperoxidase; MTTP, microsomal triglyceride transfer protein; NAFLD, non-alcoholic fatty liver disease; NAS, NAFLD activity score; NASH; NASH, nonalcoholic steatohepatitis; NF-κB, nuclear factor-κB; NOS2, nitric oxide synthase-2; NQO1, NAD(P)H:quinone oxidoreductase 1; Nrf2; Nrf2, NF-E2 p45-related factor 2; PARP, poly ADP ribose polymerase; PCR, polymerase chain reaction; PDI, protein disulfide isomerase; PERK, PRK-like endoplasmic reticulum kinase; PPARα, peroxisome proliferator-activated receptor α; PPARγ, peroxisome proliferator-activated receptor γ; PRDX6, peroxiredoxin 6; PTGR1, prostaglandin reductase-1; PTT, pyruvate tolerance test; RC, regular chow; SCAD, short-chain acyl-CoA dehydrogenase; SCD1, stearoyl-CoA desaturase-1; SFN, sulforaphane; SHP, small heterodimer partner; SLC7A11, solute carrier family 7 member 11; SREBP-1c, sterol regulatory element-binding protein-1c; TBE-31; TGFβ, transforming growth factor beta-1; TNF-α, tumor necrosis factor-α; TXN1, thioredoxin-1; TXNRD1, thioredoxin reductase-1; UPR, unfolded protein response; XBP1, X-box binding protein-1; eIf2α, eukaryotic translation initiation factor 2A; p58IPK, p58 inhibitor of the PKR kinase; qRT-PCR, quantitative reverse transcriptase PCR; α-SMA, alpha smooth muscle actin.
Figures









References
-
- Vos M.B., Lavine J.E. Dietary fructose in non-alcoholic fatty liver disease. Hepatology. 2013;57:2525–2531. - PubMed
-
- Haas J.T., Francque S., Staels B. Pathophysiology and mechanisms of nonalcoholic fatty liver disease. Annu Rev Physiol. 2016;78:181–205. - PubMed
-
- Michelotti G.A., Machado M.V., Diehl A.M. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013;10:656–665. - PubMed
-
- Tilg H., Moschen A.R. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–1846. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

