Deterministic Integration of Biological and Soft Materials onto 3D Microscale Cellular Frameworks
- PMID: 29552634
- PMCID: PMC5850936
- DOI: 10.1002/adbi.201700068
Deterministic Integration of Biological and Soft Materials onto 3D Microscale Cellular Frameworks
Abstract
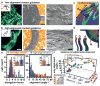
Complex 3D organizations of materials represent ubiquitous structural motifs found in the most sophisticated forms of matter, the most notable of which are in life-sustaining hierarchical structures found in biology, but where simpler examples also exist as dense multilayered constructs in high-performance electronics. Each class of system evinces specific enabling forms of assembly to establish their functional organization at length scales not dissimilar to tissue-level constructs. This study describes materials and means of assembly that extend and join these disparate systems-schemes for the functional integration of soft and biological materials with synthetic 3D microscale, open frameworks that can leverage the most advanced forms of multilayer electronic technologies, including device-grade semiconductors such as monocrystalline silicon. Cellular migration behaviors, temporal dependencies of their growth, and contact guidance cues provided by the nonplanarity of these frameworks illustrate design criteria useful for their functional integration with living matter (e.g., NIH 3T3 fibroblast and primary rat dorsal root ganglion cell cultures).
Keywords: 3D scaffolds; cellular contact guidance; compressive-assembly; direct ink writing; hydrogels.
Conflict of interest statement
Conflict of Interest The authors declare no conflict of interest.
Figures




Similar articles
-
3D-Printed pHEMA Materials for Topographical and Biochemical Modulation of Dorsal Root Ganglion Cell Response.ACS Appl Mater Interfaces. 2017 Sep 13;9(36):30318-30328. doi: 10.1021/acsami.7b06742. Epub 2017 Aug 31. ACS Appl Mater Interfaces. 2017. PMID: 28813592 Free PMC article.
-
Three-dimensional mesostructures as high-temperature growth templates, electronic cellular scaffolds, and self-propelled microrobots.Proc Natl Acad Sci U S A. 2017 Nov 7;114(45):E9455-E9464. doi: 10.1073/pnas.1713805114. Epub 2017 Oct 25. Proc Natl Acad Sci U S A. 2017. PMID: 29078394 Free PMC article.
-
Materials science. Assembly of micro/nanomaterials into complex, three-dimensional architectures by compressive buckling.Science. 2015 Jan 9;347(6218):154-9. doi: 10.1126/science.1260960. Science. 2015. PMID: 25574018
-
Emerging technologies for assembly of microscale hydrogels.Adv Healthc Mater. 2012 Mar;1(2):149-158. doi: 10.1002/adhm.201200011. Adv Healthc Mater. 2012. PMID: 23184717 Free PMC article. Review.
-
Embedded bioprinting for designer 3D tissue constructs with complex structural organization.Acta Biomater. 2022 Mar 1;140:1-22. doi: 10.1016/j.actbio.2021.11.048. Epub 2021 Dec 5. Acta Biomater. 2022. PMID: 34875360 Review.
Cited by
-
Mechanically-Guided 3D Assembly for Architected Flexible Electronics.Chem Rev. 2023 Sep 27;123(18):11137-11189. doi: 10.1021/acs.chemrev.3c00335. Epub 2023 Sep 7. Chem Rev. 2023. PMID: 37676059 Free PMC article. Review.
-
Degradation Study of Thin-Film Silicon Structures in a Cell Culture Medium.Sensors (Basel). 2022 Jan 21;22(3):802. doi: 10.3390/s22030802. Sensors (Basel). 2022. PMID: 35161547 Free PMC article.
-
Three-Dimensional Silicon Electronic Systems Fabricated by Compressive Buckling Process.ACS Nano. 2018 May 22;12(5):4164-4171. doi: 10.1021/acsnano.8b00180. Epub 2018 Apr 17. ACS Nano. 2018. PMID: 29641889 Free PMC article.
-
Integration of biological systems with electronic-mechanical assemblies.Acta Biomater. 2019 Sep 1;95:91-111. doi: 10.1016/j.actbio.2019.04.032. Epub 2019 Apr 17. Acta Biomater. 2019. PMID: 31004844 Free PMC article. Review.
-
3D Particle Free Printing of Biocompatible Conductive Hydrogel Platforms for Neuron Growth and Electrophysiological Recording.Adv Funct Mater. 2021 Apr 1;31(14):2010246. doi: 10.1002/adfm.202010246. Epub 2021 Jan 27. Adv Funct Mater. 2021. PMID: 34305503 Free PMC article.
References
-
- Yoon J, Baca AJ, Park S-I, Elvikis P, Geddes JB, Li L, Kim RH, Xiao J, Wang S, Kim T-H, Motala MJ, Ahn BY, Duoss EB, Lewis JA, Nuzzo RG, Ferreira PM, Huang Y, Rockett A, Rogers JA. Nat Mater. 2008;7:907. - PubMed
-
- Bronstein ND, Yao Y, Xu L, O’Brien E, Powers AS, Ferry VE, Alivisatos AP, Nuzzo RG. ACS Photonics. 2015;2:1576.
- Kim HS, Brueckner E, Song J, Li Y, Kim S, Lu C, Sulkin J, Choquette K, Huang Y, Nuzzo RG, Rogers JA. Proc Natl Acad Sci USA. 2011;108:10072. - PMC - PubMed
- Park SI, Xiong Y, Kim RH, Elvikis P, Meitl M, Kim DH, Wu J, Yoon J, Chang-Jae Y, Liu Z, Huang Y, Hwang KC, Ferreira P, Xiuling L, Choquette K, Rogers JA. Science. 2009;325:977. - PubMed
-
- Sun J, Ding Y, Lin NJ, Zhou J, Ro H, Soles CL, Cicerone MT, Lin-Gibson S. Biomacromolecules. 2010;11:3067. - PMC - PubMed
- Stevens MM, George JH. Science. 2005;310:1135. - PubMed
- Cukierman E, Pankov R, Stevens DR, Yamada KM. Science. 2001;294:1708. - PubMed
- Langer R, Vacanti JP. Science. 1993;260:920. - PubMed
- Curtis A, Wilkinson C. Bio-materials. 1997;18:1573. - PubMed
- Yoshinari M, Matsuzaka K, Inoue T, Oda Y, Shimono M. J Biomed Mater Res A. 2003;65A:359. - PubMed
- Clark P, Connolly P, Curtis AS, Dow JA, Wilkinson CD. Development. 1990;108:635. - PubMed
- Clark AK, Taubenberger AV, Taylor RA, Niranjan B, Chea ZY, Zotenko E, Sieh S, Pedersen JS, Norden S, Frydenberg M, Grummet JP, Pook DW, Stirzaker C, Clark SJ, Lawrence MG, Ellem SJ, Hutmacher DW, Risbridger GP Australian Prostate Cancer B. Biomaterials. 2013;34:4777. - PubMed
- Chou L, Firth JD, Uitto VJ, Brunette DM. J Cell Sci. 1995;108:1563. - PubMed
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Science. 1997;276:1425. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

