Observation of Giant Conductance Fluctuations in a Protein
- PMID: 29552645
- PMCID: PMC5851656
- DOI: 10.1088/2399-1984/aa8f91
Observation of Giant Conductance Fluctuations in a Protein
Abstract
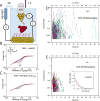
Proteins are insulating molecular solids, yet even those containing easily reduced or oxidized centers can have single-molecule electronic conductances that are too large to account for with conventional transport theories. Here, we report the observation of remarkably high electronic conductance states in an electrochemically-inactive protein, the ~200 kD αVβ3 extracelluar domain of human integrin. Large current pulses (up to nA) were observed for long durations (many ms, corresponding to many pC of charge transfer) at large gap (>5nm) distances in an STM when the protein was bound specifically by a small peptide ligand attached to the electrodes. The effect is greatly reduced when a homologous, weakly-binding protein (α4β1) is used as a control. In order to overcome the limitations of the STM, the time- and voltage-dependence of the conductance were further explored using a fixed-gap (5 nm) tunneling junction device that was small enough to trap a single protein molecule at any one time. Transitions to a high conductance (~ nS) state were observed, the protein being "on" for times from ms to tenths of a second. The high-conductance states only occur above ~ 100mV applied bias, and thus are not an equilibrium property of the protein. Nanoamp two-level signals indicate the specific capture of a single molecule in an electrode gap functionalized with the ligand. This offers a new approach to label-free electronic detection of single protein molecules. Electronic structure calculations yield a distribution of energy level spacings that is consistent with a recently proposed quantum-critical state for proteins, in which small fluctuations can drive transitions between localized and band-like electronic states.
Conflict of interest statement
Conflict of Interest SL is a cofounder of Recognition AnalytiX, licensee of this technology. SL, PZ, PP and YZ are inventors on patent applications resulting from this work.
Figures





References
-
- Nitzan A. Chemical dynamics in condensed phases. Oxford: Oxford University Press; 2006.
-
- Onuchic JN, et al. Pathway analysis of protein electron-transfer reactions. Annu Rev Biophys Biomol Struct. 1992;21:349–77. - PubMed
-
- Xiao X, Xu BQ, Tao NJ. Conductance Titration of Single Peptide Molecules. J Am Chem Soc. 2004;126:5370–5371. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
