MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis
- PMID: 29552975
- PMCID: PMC9084630
- DOI: 10.1056/NEJMoa1801993
MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis
Abstract
Background: Multiparametric magnetic resonance imaging (MRI), with or without targeted biopsy, is an alternative to standard transrectal ultrasonography-guided biopsy for prostate-cancer detection in men with a raised prostate-specific antigen level who have not undergone biopsy. However, comparative evidence is limited.
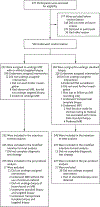
Methods: In a multicenter, randomized, noninferiority trial, we assigned men with a clinical suspicion of prostate cancer who had not undergone biopsy previously to undergo MRI, with or without targeted biopsy, or standard transrectal ultrasonography-guided biopsy. Men in the MRI-targeted biopsy group underwent a targeted biopsy (without standard biopsy cores) if the MRI was suggestive of prostate cancer; men whose MRI results were not suggestive of prostate cancer were not offered biopsy. Standard biopsy was a 10-to-12-core, transrectal ultrasonography-guided biopsy. The primary outcome was the proportion of men who received a diagnosis of clinically significant cancer. Secondary outcomes included the proportion of men who received a diagnosis of clinically insignificant cancer.
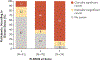
Results: A total of 500 men underwent randomization. In the MRI-targeted biopsy group, 71 of 252 men (28%) had MRI results that were not suggestive of prostate cancer, so they did not undergo biopsy. Clinically significant cancer was detected in 95 men (38%) in the MRI-targeted biopsy group, as compared with 64 of 248 (26%) in the standard-biopsy group (adjusted difference, 12 percentage points; 95% confidence interval [CI], 4 to 20; P=0.005). MRI, with or without targeted biopsy, was noninferior to standard biopsy, and the 95% confidence interval indicated the superiority of this strategy over standard biopsy. Fewer men in the MRI-targeted biopsy group than in the standard-biopsy group received a diagnosis of clinically insignificant cancer (adjusted difference, -13 percentage points; 95% CI, -19 to -7; P<0.001).
Conclusions: The use of risk assessment with MRI before biopsy and MRI-targeted biopsy was superior to standard transrectal ultrasonography-guided biopsy in men at clinical risk for prostate cancer who had not undergone biopsy previously. (Funded by the National Institute for Health Research and the European Association of Urology Research Foundation; PRECISION ClinicalTrials.gov number, NCT02380027 .).
Figures
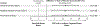
Comment in
-
MRI improves diagnosis.Nat Rev Clin Oncol. 2018 Jun;15(6):345. doi: 10.1038/s41571-018-0013-3. Nat Rev Clin Oncol. 2018. PMID: 29643470 No abstract available.
-
MRI-Targeted versus Ultrasonography-Guided Biopsy for Suspected Prostate Cancer.N Engl J Med. 2018 May 10;378(19):1835-1836. doi: 10.1056/NEJMe1804231. N Engl J Med. 2018. PMID: 29742381 No abstract available.
-
Diagnostic Pathway of Patients with a Clinical Suspicion of Prostate Cancer: Does One Size Fit All?Eur Urol. 2018 Sep;74(3):400-401. doi: 10.1016/j.eururo.2018.05.013. Epub 2018 May 24. Eur Urol. 2018. PMID: 29803581 No abstract available.
-
Magnetic Resonance Imaging Prior to First Prostate Biopsy-Are we there yet?Eur Urol. 2018 Oct;74(4):409-410. doi: 10.1016/j.eururo.2018.05.018. Epub 2018 Jun 14. Eur Urol. 2018. PMID: 29866464 No abstract available.
-
Re: MRI-targeted or Standard Biopsy for Prostate-cancer Diagnosis.Eur Urol. 2018 Oct;74(4):524-525. doi: 10.1016/j.eururo.2018.05.029. Epub 2018 Jun 7. Eur Urol. 2018. PMID: 29886028 No abstract available.
-
PRECISION delivers on the PROMIS of mpMRI in early detection.Nat Rev Urol. 2018 Sep;15(9):529-530. doi: 10.1038/s41585-018-0046-5. Nat Rev Urol. 2018. PMID: 29930321 No abstract available.
-
"Don't Let the Perfect Be the Enemy of the Good": Time to Embrace Magnetic Resonance Imaging Before First Prostate Biopsy.Eur Urol. 2018 Oct;74(4):411-412. doi: 10.1016/j.eururo.2018.06.012. Epub 2018 Jun 22. Eur Urol. 2018. PMID: 29937197 No abstract available.
-
MRI-Targeted Biopsy for Prostate-Cancer Diagnosis.N Engl J Med. 2018 Aug 9;379(6):589. doi: 10.1056/NEJMc1807507. N Engl J Med. 2018. PMID: 30091345 No abstract available.
-
MRI-Targeted Biopsy for Prostate-Cancer Diagnosis.N Engl J Med. 2018 Aug 9;379(6):589. doi: 10.1056/NEJMc1807507. N Engl J Med. 2018. PMID: 30091346 No abstract available.
-
Re: MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis.J Urol. 2018 Oct;200(4):697-699. doi: 10.1016/j.juro.2018.07.026. Epub 2018 Jul 17. J Urol. 2018. PMID: 30227580 No abstract available.
-
MRI and MRI-targeted biopsy take precedence over systematic biopsy in primary prostate cancer diagnosis.BMJ Evid Based Med. 2019 Jun;24(3):116-117. doi: 10.1136/bmjebm-2018-111081. Epub 2018 Oct 24. BMJ Evid Based Med. 2019. PMID: 30355658 No abstract available.
-
Using Multiparametric Magnetic Resonance Imaging to Shift Prostate Cancer Diagnosis Toward Clinically Significant Disease and Minimize Overdiagnosis (and Overtreatment).Int J Radiat Oncol Biol Phys. 2019 Dec 1;105(5):915-917. doi: 10.1016/j.ijrobp.2019.09.040. Int J Radiat Oncol Biol Phys. 2019. PMID: 31748142 No abstract available.
References
-
- Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017; 389: 815–22. - PubMed
-
- Hamdy FC, Donovan JL, Lane JA, et al. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016; 375: 1415–24. - PubMed
-
- Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med 2017; 377: 132–42. - PubMed
-
- Maurice MJ, Abouassaly R, Kim SP, Zhu H. Contemporary nationwide patterns of active surveillance use for prostate cancer. JAMA Intern Med 2015; 175: 1569–71. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
