Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease
- PMID: 29553157
- PMCID: PMC6494402
- DOI: 10.1002/14651858.CD006897.pub4
Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease
Abstract
Background: Current guidelines recommend that patients with acute exacerbations of chronic obstructive pulmonary disease (COPD) should be treated with systemic corticosteroid for seven to 14 days. Intermittent systemic corticosteroid use is cumulatively associated with adverse effects such as osteoporosis, hyperglycaemia and muscle weakness. Shorter treatment could reduce adverse effects.
Objectives: To compare the efficacy of short-duration (seven or fewer days) and conventional longer-duration (longer than seven days) systemic corticosteroid treatment of adults with acute exacerbations of COPD.
Search methods: Searches were carried out using the Cochrane Airways Group Specialised Register of Trials, MEDLINE and CENTRAL (Cochrane Central Register of Controlled Trials) and ongoing trials registers up to March 2017.
Selection criteria: Randomised controlled trials comparing different durations of systemic corticosteroid defined as short (i.e. seven or fewer days) or longer (i.e. longer than seven days). Other interventions-bronchodilators and antibiotics-were standardised. Studies with participants requiring assisted ventilation were excluded.
Data collection and analysis: We used standard methodological procedures as expected by The Cochrane Collaboration.
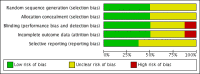
Main results: Eight studies with 582 participants met the inclusion criteria, of which five studies conducted in hospitals with 519 participants (range 28 to 296) contributed to the meta-analysis. Mean ages of study participants were 65 to 73 years, the proportion of male participants varied (58% to 84%) and COPD was classified as severe or very severe. Corticosteroid treatment was given at equivalent daily doses for three to seven days for short-duration treatment and for 10 to 15 days for longer-duration treatment. Five studies administered oral prednisolone (30 mg in four, tapered in one), and two studies provided intravenous corticosteroid treatment. Studies contributing to the meta-analysis were at low risk of selection, performance, detection and attrition bias. In four studies we did not find a difference in risk of treatment failure between short-duration and longer-duration systemic corticosteroid treatment (n = 457; odds ratio (OR) 0.72, 95% confidence interval (CI) 0.36 to 1.46)), which was equivalent to 22 fewer per 1000 for short-duration treatment (95% CI 51 fewer to 34 more). No difference in risk of relapse (a new event) was observed between short-duration and longer-duration systemic corticosteroid treatment (n = 457; OR 1.04, 95% CI 0.70 to 1.56), which was equivalent to nine fewer per 1000 for short-duration treatment (95% CI 68 fewer to 100 more). Time to the next COPD exacerbation did not differ in one large study that was powered to detect non-inferiority and compared five days versus 14 days of systemic corticosteroid treatment (n = 311; hazard ratio 0.95, 95% CI 0.66 to 1.37). In five studies no difference in the likelihood of an adverse event was found between short-duration and longer-duration systemic corticosteroid treatment (n = 503; OR 0.89, 95% CI 0.46 to 1.69, or nine fewer per 1000 (95% CI 44 fewer to 51 more)). Length of hospital stay (n = 421; mean difference (MD) -0.61 days, 95% CI -1.51 to 0.28) and lung function at the end of treatment (n = 185; MD FEV1 -0.04 L; 95% CI -0.19 to 0.10) did not differ between short-duration and longer-duration treatment.
Authors' conclusions: Information from a new large study has increased our confidence that five days of oral corticosteroids is likely to be sufficient for treatment of adults with acute exacerbations of COPD, and this review suggests that the likelihood is low that shorter courses of systemic corticosteroids (of around five days) lead to worse outcomes than are seen with longer (10 to 14 days) courses. We graded most available evidence as moderate in quality because of imprecision; further research may have an important impact on our confidence in the estimates of effect or may change the estimates. The studies in this review did not include people with mild or moderate COPD; further studies comparing short-duration systemic corticosteroid versus conventional longer-duration systemic corticosteroid for treatment of adults with acute exacerbations of COPD are required.
Conflict of interest statement
R Wood‐Baker was chief investigator in a study contributing data to this review. Data from this study were verified and analysed by JAE Walters.
Figures

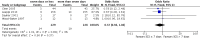
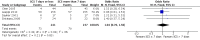


















Update of
-
Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2014 Dec 10;(12):CD006897. doi: 10.1002/14651858.CD006897.pub3. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2018 Mar 19;3:CD006897. doi: 10.1002/14651858.CD006897.pub4. PMID: 25491891 Updated.
Comment in
-
Can shorter courses of oral steroids treat acute COPD exacerbations?Drug Ther Bull. 2019 Feb;57(2):19. doi: 10.1136/dtb.2018.000058. Drug Ther Bull. 2019. PMID: 30709856 No abstract available.
References
References to studies included in this review
Chen 2005 {published and unpublished data}
-
- Chen G, Xie C. The efficacy and treatment length of oral corticosteroids in patients with acute exacerbations of chronic obstructive pulmonary disease [Abstract]. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 1966.
-
- Chen G, Xie CM, Luo YF. [The effects and therapeutic duration of oral corticosteroids in patients with acute exacerbation of chronic obstructive pulmonary diseases]. [Chinese]. Chinese Journal of Tuberculosis and Respiratory Diseases [Chung‐Hua Chieh Ho Ho Hu Hsi Tsa Chih] 2008;31(8):577‐80. - PubMed
Gomaa 2008 {published data only (unpublished sought but not used)}
-
- Gomaa M, Faramawy M, Ibrahim H. Duration of systemic corticosteroids treatment in COPD exacerbations [Abstract]. European Respiratory Society 18th Annual Congress; 2008 Oct 3‐7; Berlin. 2008:[P3601].
Leuppi 2013 {published data only}
-
- Leuppi JD, Schuetz P, Bingisser R, Bodmer M, Briel M, Drescher, et al. Short‐term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA 2013;309(21):2223‐31. - PubMed
-
- Schuetz P, Leuppi JD, Bingisser R, Bodmer M, Briel M, Drescher T, Duerring U, Henzen C, Leibbrandt Y, Maier S, Miedinger D, Mueller B, Scherr A, Schindler C, Stoeckli R, Viatte S, Garnier C, Tamm M, Rutishauser J. Prospective analysis of adrenal function in patients with acute exacerbations of COPD: the Reduction in the Use of Corticosteroids in Exacerbated COPD (REDUCE) trial. Eur J Endocrinol 2015;173(1):19‐27. - PubMed
-
- Schuetz P, Leuppi JD, Tamm M, Briel M, Bingisser R, Dürring U, et al. Short versus conventional term glucocorticoid therapy in acute exacerbation of chronic obstructive pulmonary disease—the "REDUCE" trial. Swiss Medical Weekly 2011;140(18 October 2010):w13109. - PubMed
Rahman 2004 {published data only (unpublished sought but not used)}
-
- Abdullah Al Mamun SM, Rahman S. Role of 7‐day and 14‐day courses of oral prednisolone treatment in acute exacerbation of COPD [Abstract]. Thorax 2011;66(Suppl 4):A172.
-
- Rahman M, Abdullah A, Mamun SM, Haque MD. Role of 7‐day and 14‐day courses of oral prednisolone treatment in acute exacerbation of COPD [Abstract]. Chest 2004;126(4 Suppl):839S‐a.
Salam 1998 {published data only (unpublished sought but not used)}
-
- Salam T, Akers SM, Lotano R, Arnold GK, Bartter T, Pratter MR, et al. Optimal duration of corticosteroid therapy in the treatment of exacerbations of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Suppl):A801.
Sayiner 2001 {published data only}
-
- Sayiner A, Aytemur ZA, Cirit M, Unsal I. Systemic glucocorticoids in severe exacerbations of COPD. Chest 2001;119(3):726‐30. - PubMed
Sirichana 2008 {published and unpublished data}
-
- Sirichana W, Sittipunt C, Kawkitinarong K, Wongtim S. Comparison between 5 days and 10 days of prednisolone in treatment of acute exacerbation of chronic obstructive pulmonary disease [Abstract]. Respirology 2008;13(Suppl 5):A120 [012‐01].
Wood‐Baker 1997 {published and unpublished data}
-
- Wood‐Baker R, Wilkinson J, Pearce M, Ryan G. A double‐blind, randomised, placebo‐controlled trial of corticosteroids for acute exacerbations of chronic obstructive pulmonary disease [Abstract]. Australian & New Zealand Journal of Medicine 1998;28:262.
References to studies excluded from this review
Aaron 2003 {published data only (unpublished sought but not used)}
-
- Aaron SD, Vandemheen KL, Hebert P, Dales R, Stiell IG, Ahuja J, et al. Outpatient oral prednisone after emergency treatment of chronic obstructive pulmonary disease [comment]. New England Journal of Medicine 2003;348(26):2618‐25. - PubMed
Abroug 2014 {published data only}
-
- Abroug F, Ouanes‐Besbes L, Fkih‐Hassen M, Ouanes I, Ayed S, Dachraoui F, Brochard L, Elatrous S. Prednisone in COPD exacerbation requiring ventilatory support: an open‐label randomised evaluation. Eur Respir J 2014;43(3):717‐24. - PubMed
Albert 1980 {published data only}
-
- Albert RK, Martin TR, Lewis SW. Controlled clinical trial of methylprednisolone in patients with chronic bronchitis and acute respiratory insufficiency. Annals of Internal Medicine 1980;92(6):753‐8. - PubMed
Albert 2008 {published data only}
-
- Albert P, Calverley P. A PEACE‐ful solution to COPD exacerbations? [comment]. Lancet 2008;371(9629):1975‐6. - PubMed
Ardestani 2017 {published data only}
Brusse‐Keizer 2014 {published data only}
Ceviker 2014 {published data only}
-
- Ceviker Y, Sayiner A. Comparison of two systemic steroid regimens for the treatment of COPD exacerbations. Pulm Pharmacol Ther Apr 2014;27(2):179‐83. - PubMed
Cordero 1996 {published data only}
-
- Cordero PJ, Benlloch E, Solè A, Morales P, Menèndez R, Cebri·NJ. Corticosteroid therapy for COPD exacerbations at inpatient settings: a controlled‐randomized study. Archivos de Bronconeumologia 1996;32(Suppl 2):17.
-
- Cordero PJ, Solè A, Benlloch E, Morales P, Vallterra J, Menèndez R, et al. A randomized controlled study of prednisone in outpatients with acute exacerbation of COPD. European Respiratory Journal Supplement 1996;9 Suppl 23:110s‐1s.
Courtney 2003 {published data only}
-
- Courtney AU. Oral prednisone prevents relapse in COPD exacerbations. Journal of Family Practice 2003;52(10):762‐4. - PubMed
Davies 1999 {published data only}
-
- Davies L, Angus R, Mand Calverley PMA. A prospective, randomised, double‐blind, placebo controlled study of oral corticosteroids in patients admitted with normocapnic acute exacerbations of chronic obstructive pulmonary disease (COPD). Thorax 1997;52(Suppl 6):A5 S18.
-
- Davies L, Angus RM, Calverley PM. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet 1999;354(9177):456‐60. - PubMed
De Jong 2007 {published data only}
-
- Jong YP, Uil M, Grotjohan P, Postma S, Kerstjens M, Berg JWK. A comparison of intravenous versus oral administration of corticosteroids in the treatment of patients admitted to the hospital with an exacerbation of COPD [Abstract]. European Respiratory Journal 2004;24(Suppl 48):64s.
-
- Jong YP, Uil SM, Grotjohan HP, Postma DS, Kerstjens HA, Berg JW. Oral or IV prednisolone in the treatment of COPD exacerbations: a randomized, controlled, double‐blind study.[see comment]. Comment in: Chest. 2007 Dec;132(6):1728‐9; PMID: 18079214. Chest 2007;132(6):1741‐7. - PubMed
Ding 2016 {published data only}
-
- Ding Z, Li X, Lu Y, Rong G, Yang R, Zhang R, Wang G, Wei X, Ye Y, Qian Z, Liu H, Zhu D, Zhou R, Zhu K, Ni R, Xia K, Luo N, Pei C. A randomized, controlled multicentric study of inhaled budesonide and intravenous methylprednisolone in the treatment on acute exacerbation of chronic obstructive pulmonary disease. Respiratory medicine 2016;121:39‐47. - PubMed
GlaxoSmithKline 2005 {published data only}
-
- GlaxoSmithKline. A single centre, randomised, double‐blind, parallel group study to compare the efficacy of nebulised fluticasone propionate 2 mg twice daily (bd) with placebo in patients with moderate to severe chronic obstructive pulmonary disease. GlaxoSmithKline Clinical Trial Register 2005.
Kalkoti 2014 {published data only (unpublished sought but not used)}
-
- Kalkoti V, Kanani NJ. A prospective study for evaluating the clinical efficacy of methylxanthines in patients of acute exacerbation of chronic obstructive pulmonary disease (AECOPD) admitted in a tertiary care hospital. Indian journal of pharmacology 2014;46(7 SUPPL. 1):S25.
Li 2003 {published data only}
-
- Li H, He G, Chu H, Zhao L, Yu H. A step‐wise application of methylprednisolone versus dexamethasone in the treatment of acute exacerbations of COPD. Respirology 2003;8(2):199‐204. - PubMed
-
- Li HP, He GJ, Chu HQ. The application of methylprednisolone in acute exacerbations of COPD. European Respiratory Journal 2000;16(Suppl 31):50s.
Makarova 2016 {published data only (unpublished sought but not used)}
-
- Makarova EV, Varvarina GN, Menkov NV, Tsapaeva MY, Lazareva ES, Kazatskaya ZhA, Novikov VV, Karaulov AV. Nebulized budesonide in the treatment of exacerbations of chronic obstructive pulmonary disease: Efficacy, safety, and effects on the serum levels of soluble differentiation molecules. Terapevticheskii arkhi 2016;88(3):24‐31. - PubMed
McCrory 2000 {published data only}
-
- McCrory DC, Hasselblad V. The results of a randomized controlled trial of hydrocortisone in acute exacerbation of COPD. American Journal of Emergency Medicine 2000;18(1):122. - PubMed
Odonchimeg 2015 {published data only}
-
- Odonchimeg P, Ichinnorov D, Sarantuyaa Zh, Tumur‐Ochir Ts, Choyzhamts G. Efficacy of different regimens of steroid therapy in patients with exacerbation of chronic obstructive pulmonary disease. Pulmonologiya 2015;25(1):58‐63.
Sharma 2014 {published data only}
-
- Sharma N, Venado A, Morrison J. Protocol‐based treatment of septic shock, fibrinolysis for submassive pulmonary embolism, and use of corticosteroids in acute exacerbations of chronic obstructive pulmonary disease requiring mechanical ventilation. Am J Respir Crit Care Med 2014;190(7):827‐8. - PubMed
Sun 2015 {published data only}
Sun 2015a {published data only}
-
- Sun X, He Z, Zhang J, Deng J, Bai J, Li M, Zhong X. Compare the efficacy of inhaled budesonide and systemic methylprednisolone on systemic inflammation of AECOPD. Pulmonary pharmacology & therapeutics 2015;31:111‐116. - PubMed
Woods 2014 {published data only}
Yilmazel Ucar 2014 {published data only}
References to ongoing studies
Leuppi 2017 {unpublished data only}
-
- Leuppi J. Reduction of Corticosteroid Use in Outpatient Treatment of Exacerbated COPD (RECUT). Clinicaltrials.gov 2015:NCT02386735.
Reid 2010 {unpublished data only}
-
- Reid D, Soltani A, Wood‐Baker R, Walters EH. Personal correspondence 2010.
Additional references
Agusti 2010
Anthonisen 1987
-
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Annals of Internal Medicine 1987;106(2):196‐204. - PubMed
Bathoorn 2008
Buist 2007
-
- Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (the BOLD study): a population‐based prevalence study. Lancet 2007;370(9589):741‐50. - PubMed
De Vries 2007
-
- Vries F, Bracke M, Leufkens HGM, Lammers JWJ, Cooper C, Staa TP. Fracture risk with intermittent high‐dose oral glucocorticoid therapy. Arthritis and Rheumatism 2007;56(1):208‐14. - PubMed
Decramer 1994
-
- Decramer M, Lacquet LM, Fagard R, Rogiers P. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. American Journal of Respiratory and Critical Care Medicine 1994;150(1):11‐6. - PubMed
Deeks 2008
-
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. www.cochrane‐handbook.org. The Cochrane Collaboration, 2008.
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. - PubMed
Donaldson 2002
Donaldson 2005
-
- Donaldson GC, Wilkinson TMA, Hurst JR, Perera WR, Wedzicha JA. Exacerbations and time spent outdoors in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2005;171:446‐52. - PubMed
Donaldson 2006
Effing 2009
-
- Effing TW, Kerstjens HAM, Monninkhof EM, Valk PDLPM, Wouters EFM, Postma DS, et al. Definitions of exacerbations: does it really matter in clinical trials on COPD?. Chest 2009;136(3):918‐23. - PubMed
Evans 2002
-
- Evans AT, Husain S, Durairaj L, Sadowski LS, Charles‐Damte M, Wang Y. Azithromycin for acute bronchitis: a randomised, double‐blind, controlled trial. Lancet 2002;359(9318):1648‐54. - PubMed
Falk 2008
GOLD 2014
-
- GOLD. Global Strategy for the Diagnosis, Management and Prevention of COPD, 2014. http://www.goldcopd.org/.
Groenewegen 2001
-
- Groenewegen K, Schols A, Wouters E. Prognosis after hospitalization for acute exacerbations of COPD. European Respiratory Journal 2001;8(Suppl 33):209s.
Hardin 2014
Higgins 2003
Higgins 2011
-
- Higgins J, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hurst 2010
-
- Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal‐Singer R, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. New England Journal of Medicine 2010;363(12):1128‐38. - PubMed
Jones 2014
-
- Jones PW, Lamarca R, Chuecos F, Singh D, Agusti A, Bateman ED, et al. Characterisation and impact of reported and unreported exacerbations: results from ATTAIN. European Respiratory Journal 2014;epub ahead of print:doi:10.1183/09031936.00038814. - PubMed
Kiser 2014
-
- Kiser TH, Allen RR, Valuck RJ, Moss M, Vandivier RW. Outcomes associated with corticosteroid dosage in critically ill patients with acute exacerbations of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2014;189(9):1052‐64. - PubMed
Leidy 2010
-
- Leidy NK, Wilcox TK, Jones PW, Murray L, Winnette R, Howard K, et al. Development of the EXAcerbations of chronic obstructive pulmonary disease Tool (EXACT): a patient‐reported outcome (PRO) measure. Value in Health 2010;13(8):965‐75. - PubMed
Leidy 2011
-
- Leidy NK, Wilcox TK, Jones PW, Roberts L, Powers JH, Sethi S. Standardizing measurement of chronic obstructive pulmonary disease exacerbations. American Journal of Respiratory and Critical Care Medicine 2011;183(3):323‐9. - PubMed
Leidy 2014
-
- Leidy NK, Murray LT, Jones P, Sethi S. Performance of the EXAcerbations of Chronic Pulmonary Disease Tool Patient‐reported Outcome Measure in three clinical trials of chronic obstructive pulmonary disease. Annals of the American Thoracic Society 2014;11(3):316‐25. - PubMed
Lindenauer 2010
-
- Lindenauer PK, Pekow PS, Lahti MC, Lee Y, Benjamin EM, Rothberg MB. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA 2010;303(23):2359‐67. - PubMed
Lopez 2006
-
- Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, et al. Chronic obstructive pulmonary disease: current burden and future projections. European Respiratory Journal 2006;27(2):397‐412. - PubMed
Majumdar 2010
-
- Majumdar SR, Villa‐Roel C, Lyons KJ, Rowe BH. Prevalence and predictors of vertebral fracture in patients with chronic obstructive pulmonary disease. Respiratory Medicine 2010;104(2):260‐6. - PubMed
McEvoy 1996
-
- McEvoy CE, Niewoehner DE. Adverse effects of corticosteroid therapy for COPD. Chest 1997;111:732‐43. - PubMed
McKenzie 2003
-
- McKenzie DK, Frith PA, Burdon JG, Town GI. The COPDX Plan: Australian and New Zealand guidelines for the management of chronic obstructive pulmonary disease 2003. Medical Journal of Australia 2003;178(Suppl):S7‐S39. - PubMed
Murray 1997
-
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990‐2020: global burden of disease study. Lancet 1997;349(9064):1498‐504. - PubMed
NICE 2010
-
- National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease: national clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care [update 2010]. http://guidance.nice.org.uk/CG101 (accessed 18 July 2011).
Oostenbrink 2004
-
- Oostenbrink JB, Rutten‐van Molken MP. Resource use and risk factors in high‐cost exacerbations of COPD. Respiratory Medicine 2004;98:883‐91. - PubMed
Papi 2006
-
- Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. American Journal of Respiratory and Critical Care Medicine 2006;173(10):1114‐21. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rodriguez‐Roisin 2000
-
- Rodriguez‐Roisin R. Toward a consensus definition for COPD exacerbations. Chest 2000;117(5 Suppl 2):398S‐401S. - PubMed
Schermer 2002
-
- Schermer T, Chavannes N, Saris C, Akkermans R, Schayck C, Weel C. Exacerbations and associated health care cost in patients with chronic obstructive pulmonary disease in general practice. Results from the COOPT trial. European Respiratory Journal 2002;20(Suppl 38):398s.
Seemungal 2000
-
- Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2000;161(5):1608‐13. - PubMed
Sethi 2002
-
- Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. New England Journal of Medicine 2002;347(7):465‐71. - PubMed
Sethi 2008
-
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. New England Journal of Medicine 2008;359(22):2355‐65. - PubMed
Sherk 2000
Soler‐Cataluna 2005
Suissa 2012
Sullivan 2000
-
- Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest 2000;117:5s‐9s. - PubMed
Therapeutic Guidelines 2009
-
- Therapeutic Guidelines Ltd. Therapeutic Guidelines: Respiratory. Version 4. Melbourne: Therapeutic Guidelines Ltd, 2009.
Vestergaard 2007
-
- Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk in patients with chronic lung diseases treated with bronchodilator drugs and inhaled and oral corticosteroids. Chest 2007;132(5):1599‐607. [PUBMED: 17890464 ] - PubMed
Walters 2011
Walters 2014
Weatherall 2009
-
- Weatherall M, Travers J, Shirtcliffe PM, Marsh SE, Williams MV, Nowitz MR, et al. Distinct clinical phenotypes of airways disease defined by cluster analysis. European Respiratory Journal 2009;34(4):812‐8. - PubMed
Wedzicha 2000
Wilkinson 2004
-
- Wilkinson TM, Donaldson GC, Hurst JR, Seemungal TA, Wedzicha JA. Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2004;169(12):1298‐303. - PubMed
Wouters 2003
-
- Wouters EFM. Economic analysis of the confronting COPD survey: an overview of results. Respiratory Medicine 2003;97(Suppl 3):S3‐S14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

