Artemisinin-Resistant Plasmodium falciparum with High Survival Rates, Uganda, 2014-2016
- PMID: 29553316
- PMCID: PMC5875287
- DOI: 10.3201/eid2404.170141
Artemisinin-Resistant Plasmodium falciparum with High Survival Rates, Uganda, 2014-2016
Abstract
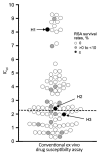
Because ≈90% of malaria cases occur in Africa, emergence of artemisinin-resistant Plasmodium falciparum in Africa poses a serious public health threat. To assess emergence of artemisinin-resistant parasites in Uganda during 2014-2016, we used the recently developed ex vivo ring-stage survival assay, which estimates ring-stage-specific P. falciparum susceptibility to artemisinin. We conducted 4 cross-sectional surveys to assess artemisinin sensitivity in Gulu, Uganda. Among 194 isolates, survival rates (ratio of viable drug-exposed parasites to drug-nonexposed controls) were high (>10%) for 4 isolates. Similar rates have been closely associated with delayed parasite clearance after drug treatment and are considered to be a proxy for the artemisinin-resistant phenotype. Of these, the PfKelch13 mutation was observed in only 1 isolate, A675V. Population genetics analysis suggested that these possibly artemisinin-resistant isolates originated in Africa. Large-scale surveillance of possibly artemisinin-resistant parasites in Africa would provide useful information about treatment outcomes and help regional malaria control.
Keywords: Artemisinin; Plasmodium falciparum; Uganda; antimicrobial resistance; drug resistance; malaria; parasites.
Figures


References
-
- World Health Organization. World malaria report 2017. Geneva: The Organization; 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

