Genomic Surveillance of 4CMenB Vaccine Antigenic Variants among Disease-Causing Neisseria meningitidis Isolates, United Kingdom, 2010-2016
- PMID: 29553330
- PMCID: PMC5875271
- DOI: 10.3201/eid2404.171480
Genomic Surveillance of 4CMenB Vaccine Antigenic Variants among Disease-Causing Neisseria meningitidis Isolates, United Kingdom, 2010-2016
Abstract
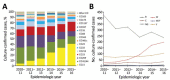
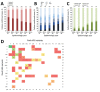
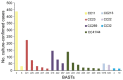
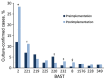
In September 2015, 4CMenB meningococcal vaccine was introduced into the United Kingdom infant immunization program without phase 3 trial information. Understanding the effect of this program requires enhanced surveillance of invasive meningococcal disease (IMD) Neisseria meningitidis isolates and comparison with prevaccination isolates. Bexsero Antigen Sequence Types (BASTs) were used to analyze whole-genome sequences of 3,073 prevaccine IMD N. meningitidis isolates obtained during 2010-2016. Isolates exhibited 803 BASTs among 31 clonal complexes. Frequencies of antigen peptide variants were factor H binding protein 1, 13.4%; Neisserial heparin-binding antigen 2, 13.8%; Neisseria adhesin A 8, 0.8%; and Porin A-VR2:P1.4,10.9%. In 2015-16, serogroup B isolates showed the highest proportion (35.7%) of exact matches to >1 Bexsero components. Serogroup W isolates showed the highest proportion (93.9%) of putatively cross-reactive variants of Bexsero antigens. Results highlighted the likely role of cross-reactive antigens. BAST surveillance of meningococcal whole-genome sequence data is rapid, scalable, and portable and enables international comparisons of isolates.
Keywords: 4CMenB; Bexsero; IMD; MLST; Neisseria meningitidis; United Kingdom; bacteria; epidemiologic year; genomic surveillance; invasive meningococcal disease; meningitis/encephalitis; meningococcal vaccines; meningococci; molecular epidemiology; multilocus sequence typing; vaccine antigenic variants; vaccines; whole-genome sequencing.
Figures
Similar articles
-
Distribution of Bexsero® Antigen Sequence Types (BASTs) in invasive meningococcal disease isolates: Implications for immunisation.Vaccine. 2016 Sep 7;34(39):4690-4697. doi: 10.1016/j.vaccine.2016.08.015. Epub 2016 Aug 9. Vaccine. 2016. PMID: 27521232 Free PMC article.
-
Characterization of invasive Neisseria meningitidis strains from Québec, Canada, during a period of increased serogroup B disease, 2009-2013: phenotyping and genotyping with special emphasis on the non-carbohydrate protein vaccine targets.BMC Microbiol. 2015 Jul 25;15:143. doi: 10.1186/s12866-015-0469-6. BMC Microbiol. 2015. PMID: 26204985 Free PMC article.
-
Genomic Characterization of Invasive Meningococcal Serogroup B Isolates and Estimation of 4CMenB Vaccine Coverage in Finland.mSphere. 2020 Sep 16;5(5):e00376-20. doi: 10.1128/mSphere.00376-20. mSphere. 2020. PMID: 32938694 Free PMC article.
-
Meningococcal Antigen Typing System Development and Application to the Evaluation of Effectiveness of Meningococcal B Vaccine and Possible Use for Other Purposes.J Immunol Res. 2015;2015:353461. doi: 10.1155/2015/353461. Epub 2015 Aug 17. J Immunol Res. 2015. PMID: 26351645 Free PMC article. Review.
-
The Development of a Vaccine Against Meningococcus B Using Reverse Vaccinology.Front Immunol. 2019 Apr 16;10:751. doi: 10.3389/fimmu.2019.00751. eCollection 2019. Front Immunol. 2019. PMID: 31040844 Free PMC article. Review.
Cited by
-
Neisseria meningitidis: using genomics to understand diversity, evolution and pathogenesis.Nat Rev Microbiol. 2020 Feb;18(2):84-96. doi: 10.1038/s41579-019-0282-6. Epub 2019 Nov 8. Nat Rev Microbiol. 2020. PMID: 31705134 Review.
-
'Be on the TEAM' Study (Teenagers Against Meningitis): protocol for a controlled clinical trial evaluating the impact of 4CMenB or MenB-fHbp vaccination on the pharyngeal carriage of meningococci in adolescents.BMJ Open. 2020 Oct 22;10(10):e037358. doi: 10.1136/bmjopen-2020-037358. BMJ Open. 2020. PMID: 33093030 Free PMC article.
-
Genomic surveillance of Neisseria meningitidis serogroup B invasive strains: Diversity of vaccine antigen types, Brazil, 2016-2018.PLoS One. 2020 Dec 21;15(12):e0243375. doi: 10.1371/journal.pone.0243375. eCollection 2020. PLoS One. 2020. PMID: 33347452 Free PMC article.
-
Invasive meningococcal disease in Shanghai, China from 1950 to 2016: implications for serogroup B vaccine implementation.Sci Rep. 2018 Aug 17;8(1):12334. doi: 10.1038/s41598-018-30048-x. Sci Rep. 2018. PMID: 30120257 Free PMC article.
-
Exploiting Real-Time Genomic Surveillance Data To Assess 4CMenB Meningococcal Vaccine Performance in Scotland, 2015 to 2022.mBio. 2023 Apr 25;14(2):e0049923. doi: 10.1128/mbio.00499-23. Epub 2023 Apr 10. mBio. 2023. PMID: 37036356 Free PMC article.
References
-
- Salisbury D, Ramsay MK. Immunisation against infectious disease. London: The Stationery Office; 2006.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

