Sox2 haploinsufficiency primes regeneration and Wnt responsiveness in the mouse cochlea
- PMID: 29553487
- PMCID: PMC5873847
- DOI: 10.1172/JCI97248
Sox2 haploinsufficiency primes regeneration and Wnt responsiveness in the mouse cochlea
Abstract
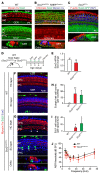
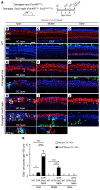
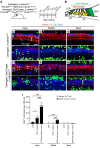
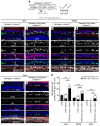
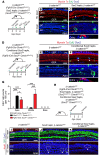
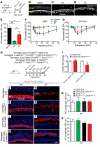
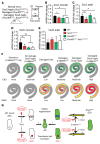
During development, Sox2 is indispensable for cell division and differentiation, yet its roles in regenerating tissues are less clear. Here, we used combinations of transgenic mouse models to reveal that Sox2 haploinsufficiency (Sox2haplo) increases rather than impairs cochlear regeneration in vivo. Sox2haplo cochleae had delayed terminal mitosis and ectopic sensory cells, yet normal auditory function. Sox2haplo amplified and expanded domains of damage-induced Atoh1+ transitional cell formation in neonatal cochlea. Wnt activation via β-catenin stabilization (β-cateninGOF) alone failed to induce proliferation or transitional cell formation. By contrast, β-cateninGOF caused proliferation when either Sox2haplo or damage was present, and transitional cell formation when both were present in neonatal, but not mature, cochlea. Mechanistically, Sox2haplo or damaged neonatal cochleae showed lower levels of Sox2 and Hes5, but not of Wnt target genes. Together, our study unveils an interplay between Sox2 and damage in directing tissue regeneration and Wnt responsiveness and thus provides a foundation for potential combinatorial therapies aimed at stimulating mammalian cochlear regeneration to reverse hearing loss in humans.
Keywords: Cell Biology; Mouse models; Mouse stem cells; Neurodegeneration; Neuroscience.
Conflict of interest statement
Figures

Similar articles
-
Extensive Supporting Cell Proliferation and Mitotic Hair Cell Generation by In Vivo Genetic Reprogramming in the Neonatal Mouse Cochlea.J Neurosci. 2016 Aug 17;36(33):8734-45. doi: 10.1523/JNEUROSCI.0060-16.2016. J Neurosci. 2016. PMID: 27535918 Free PMC article.
-
Extent of genetic and epigenetic factor reprogramming via a single viral vector construct in deaf adult mice.Hear Res. 2025 Mar;457:109170. doi: 10.1016/j.heares.2024.109170. Epub 2024 Dec 20. Hear Res. 2025. PMID: 39848037
-
A dual function for canonical Wnt/β-catenin signaling in the developing mammalian cochlea.Development. 2012 Dec 1;139(23):4395-404. doi: 10.1242/dev.080358. Development. 2012. PMID: 23132246 Free PMC article.
-
Therapeutic Potential of Wnt and Notch Signaling and Epigenetic Regulation in Mammalian Sensory Hair Cell Regeneration.Mol Ther. 2019 May 8;27(5):904-911. doi: 10.1016/j.ymthe.2019.03.017. Epub 2019 Mar 30. Mol Ther. 2019. PMID: 30982678 Free PMC article. Review.
-
Atoh1 regulation in the cochlea: more than just transcription.J Zhejiang Univ Sci B. 2019 Feb.;20(2):146-155. doi: 10.1631/jzus.B1600438. Epub 2017 Jul 13. J Zhejiang Univ Sci B. 2019. PMID: 29770645 Free PMC article. Review.
Cited by
-
The Notch Ligand Jagged1 Is Required for the Formation, Maintenance, and Survival of Hensen's Cells in the Mouse Cochlea.J Neurosci. 2020 Dec 2;40(49):9401-9413. doi: 10.1523/JNEUROSCI.1192-20.2020. Epub 2020 Oct 30. J Neurosci. 2020. PMID: 33127852 Free PMC article.
-
Sox2 is required in supporting cells for normal levels of vestibular hair cell regeneration in adult mice.Hear Res. 2022 Dec;426:108642. doi: 10.1016/j.heares.2022.108642. Epub 2022 Oct 27. Hear Res. 2022. PMID: 36334348 Free PMC article.
-
Dual regulation of planar polarization by secreted Wnts and Vangl2 in the developing mouse cochlea.Development. 2020 Oct 5;147(19):dev191981. doi: 10.1242/dev.191981. Development. 2020. PMID: 32907846 Free PMC article.
-
Developmental GAD2 Expression Reveals Progenitor-like Cells with Calcium Waves in Mammalian Crista Ampullaris.iScience. 2020 Aug 21;23(8):101407. doi: 10.1016/j.isci.2020.101407. Epub 2020 Jul 24. iScience. 2020. PMID: 32771977 Free PMC article.
-
Sox9 Inhibits Cochlear Hair Cell Fate by Upregulating Hey1 and HeyL Antagonists of Atoh1.Cells. 2023 Aug 25;12(17):2148. doi: 10.3390/cells12172148. Cells. 2023. PMID: 37681879 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

