Systemic hyperfibrinolysis after trauma: a pilot study of targeted proteomic analysis of superposed mechanisms in patient plasma
- PMID: 29554044
- PMCID: PMC5970039
- DOI: 10.1097/TA.0000000000001878
Systemic hyperfibrinolysis after trauma: a pilot study of targeted proteomic analysis of superposed mechanisms in patient plasma
Abstract
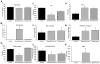
Background: Viscoelastic measurements of hemostasis indicate that 20% of seriously injured patients exhibit systemic hyperfibrinolysis, with increased early mortality. These patients have normal clot formation with rapid clot lysis. Targeted proteomics was applied to quantify plasma proteins from hyperfibrinolytic (HF) patients to elucidate potential pathophysiology.
Methods: Blood samples were collected in the field or at emergency department arrival and thrombelastography (TEG) was used to characterize in vitro clot formation under native and tissue plasminogen activator (tPA)-stimulated conditions. Ten samples were taken from injured patients exhibiting normal lysis time at 30 min (Ly30), "eufibrinolytic" (EF), 10 from HF patients, defined as tPA-stimulated TEG Ly30 >50%, and 10 from healthy controls. Trauma patient samples were analyzed by targeted proteomics and ELISA assays for specific coagulation proteins.
Results: HF patients exhibited increased plasminogen activation. Thirty-three proteins from the HF patients were significantly decreased compared with healthy controls and EF patients; 17 were coagulation proteins with anti-protease consumption (p < 0.005). The other 16 decreased proteins indicate activation of the alternate complement pathway, depletion of carrier proteins, and four glycoproteins. CXC7 was elevated in all injured patients versus healthy controls (p < 0.005), and 35 proteins were unchanged across all groups (p > 0.1 and fold change of concentrations of 0.75-1.3).
Conclusion: HF patients had significant decreases in specific proteins and support mechanisms known in trauma-induced hyperfibrinolysis and also unexpected decreases in coagulation factors, factors II, X, and XIII, without changes in clot formation (SP, R times, or angle). Decreased clot stability in HF patients was corroborated with tPA-stimulated TEGs.
Level of evidence: Prognostic, level III.
Conflict of interest statement
The authors have no conflict of interest with the submitted work.
Figures

Similar articles
-
Overwhelming tPA release, not PAI-1 degradation, is responsible for hyperfibrinolysis in severely injured trauma patients.J Trauma Acute Care Surg. 2016 Jan;80(1):16-23; discussion 23-5. doi: 10.1097/TA.0000000000000885. J Trauma Acute Care Surg. 2016. PMID: 26491796 Free PMC article.
-
Plasmin thrombelastography rapidly identifies trauma patients at risk for massive transfusion, mortality, and hyperfibrinolysis: A diagnostic tool to resolve an international debate on tranexamic acid?J Trauma Acute Care Surg. 2020 Dec;89(6):991-998. doi: 10.1097/TA.0000000000002941. J Trauma Acute Care Surg. 2020. PMID: 33230046
-
Fibrinolysis shutdown is associated with a fivefold increase in mortality in trauma patients lacking hypersensitivity to tissue plasminogen activator.J Trauma Acute Care Surg. 2017 Dec;83(6):1014-1022. doi: 10.1097/TA.0000000000001718. J Trauma Acute Care Surg. 2017. PMID: 29190254 Free PMC article.
-
Fibrinolysis Shutdown in Trauma: Historical Review and Clinical Implications.Anesth Analg. 2019 Sep;129(3):762-773. doi: 10.1213/ANE.0000000000004234. Anesth Analg. 2019. PMID: 31425218 Free PMC article. Review.
-
Temporal Changes in Fibrinolysis following Injury.Semin Thromb Hemost. 2020 Mar;46(2):189-198. doi: 10.1055/s-0039-1701016. Epub 2020 Mar 11. Semin Thromb Hemost. 2020. PMID: 32160644 Review.
Cited by
-
28-day thawed plasma maintains α2 -antiplasmin levels and inhibits tPA-induced fibrinolysis.Vox Sang. 2021 Feb;116(2):181-189. doi: 10.1111/vox.12997. Epub 2020 Sep 7. Vox Sang. 2021. PMID: 32894784 Free PMC article.
-
Proteomics of Coagulopathy Following Injury Reveals Limitations of Using Laboratory Assessment to Define Trauma-Induced Coagulopathy to Predict Massive Transfusion.Ann Surg Open. 2022 Jun;3(2):e167. doi: 10.1097/as9.0000000000000167. Epub 2022 May 25. Ann Surg Open. 2022. PMID: 36177090 Free PMC article.
-
Maintaining the balance: the critical role of plasmin activity in orthopedic surgery injury response.J Thromb Haemost. 2023 Oct;21(10):2653-2665. doi: 10.1016/j.jtha.2023.08.002. Epub 2023 Aug 8. J Thromb Haemost. 2023. PMID: 37558131 Free PMC article. Review.
-
Complement as driver of systemic inflammation and organ failure in trauma, burn, and sepsis.Semin Immunopathol. 2021 Dec;43(6):773-788. doi: 10.1007/s00281-021-00872-x. Epub 2021 Jun 30. Semin Immunopathol. 2021. PMID: 34191093 Free PMC article. Review.
-
An engineered activated factor V for the prevention and treatment of acute traumatic coagulopathy and bleeding in mice.Blood Adv. 2022 Feb 8;6(3):959-969. doi: 10.1182/bloodadvances.2021005257. Blood Adv. 2022. PMID: 34861695 Free PMC article.
References
-
- Brohi K, Cohen MJ, Ganter MT, Schultz MJ, Levi M, Mackersie RC, Pittet JF. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma. 2008 May;64(5):1211–1217. - PubMed
-
- Cotton BA, Harvin JA, Kostousouv V, Minei KM, Radwan ZA, Schochl H, Wade CE, Holcomb JB, Matijevic N. Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. J Trauma Acute Care Surg. 2012 Aug;73(2):365–370. - PubMed
-
- Moore HB, Moore EE, Gonzalez E, Chapman MP, Chin TL, Silliman CC, Banerjee A, Sauaia A. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. 2014 Dec;77(6):811–817. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

