Characteristics of Pulmonary Vascular Remodeling in a Novel Model of Shunt-Associated Pulmonary Arterial Hypertension
- PMID: 29554080
- PMCID: PMC5870112
- DOI: 10.12659/msm.905654
Characteristics of Pulmonary Vascular Remodeling in a Novel Model of Shunt-Associated Pulmonary Arterial Hypertension
Abstract
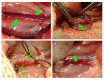
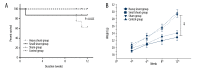
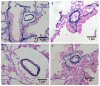
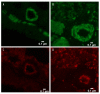
BACKGROUND Establishing a shunt-induced pulmonary arterial hypertension (PAH) model in mice would be of great scientific value, but no such models have been reported to date. Here, we established a shunt-associated PAH in mice to investigate the characteristics of pulmonary vascular remodeling, which provides a new platform for the in-depth study of PAH associated with congenital heart disease (CHD). MATERIAL AND METHODS Eighty mice were randomly divided into the heavy shunt group (n=32), the small shunt group (n=32), the sham operation group (n=8), and the control group (n=8). The septum of the abdominal aorta and inferior vena cava was cut directly to create a heavy abdominal aortocaval shunt. Pulmonary artery pressure, right ventricular hypertrophy index, and lung tissue morphology were evaluated in the 4th, 6th, 8th, and 12th weeks in the shunt groups. RESULTS Shunt-associated PAH by abdominal aortocaval shunt in mice was successfully established. The shunt patency rate was significantly higher in the heavy shunt group. Significant differences were observed between the heavy shunt group and other groups in terms of pulmonary artery pressure and the right ventricular hypertrophy index. Tissue sections revealed a thickened pulmonary intimal layer and muscular layer and stenosis of the lumen in the shunt groups. Immunofluorescent assay results showed significant proliferations of PAH smooth muscle cells and endothelial cells, consistent with the clinical pulmonary vascular remodeling seen in human patients with severe PAH. CONCLUSIONS Shunt-associated PAH established by directly cutting the septum between the abdominal aorta and inferior vena cava is a stable and reliable model for research on PAH associated with CHD.
Conflict of interest statement
None.
Figures

References
-
- Lowe BS, Therrien J, Ionescu-Ittu R, et al. Diagnosis of pulmonary hypertension in the congenital heart disease adult population impact on outcomes. J Am Coll Cardiol. 2011;58:538–46. - PubMed
-
- Diller GP, Gatzoulis MA. Pulmonary vascular disease in adults with congenital heart disease. Circulation. 2007;115:1039–50. - PubMed
-
- Schulze-Neick I, Deanfield J. Pulmonary arterial hypertension in adults with congenital heart disease: General overview of disease mechanisms. Advances in Pulmonary Hypertension Autumn. 2007;6:121–25.
-
- Canniere DD, Stefanidis C, Brimioulle S, Naeije R. Effects of a chronic aortopulmonary shunt on pulmonary hemodynamics in piglets. J Appl Physiol. 1994;77:1591–96. - PubMed
-
- van Albada ME, Schoemaker RG, Kemna MS, et al. The role of increased pulmonary blood flow in pulmonary arterial hypertension. Eur Respir J. 2005;26:487–93. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

