KCa3.1 ion channel: A novel therapeutic target for corneal fibrosis
- PMID: 29554088
- PMCID: PMC5858751
- DOI: 10.1371/journal.pone.0192145
KCa3.1 ion channel: A novel therapeutic target for corneal fibrosis
Abstract
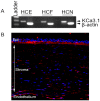
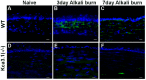
Vision impairment from corneal fibrosis is a common consequence of irregular corneal wound healing after injury. Intermediate-conductance calmodulin/calcium-activated K+ channels 3.1 (KCa3.1) play an important role in cell cycle progression and cellular proliferation. Proliferation and differentiation of corneal fibroblasts to myofibroblasts can lead to corneal fibrosis after injury. KCa3.1 has been shown in many non-ocular tissues to promote fibrosis, but its role in corneal fibrosis is still unknown. In this study, we characterized the expression KCa3.1 in the human cornea and its role in corneal wound healing in vivo using a KCa3.1 knockout (KCa3.1-/-) mouse model. Additionally, we tested the hypothesis that blockade of KCa3.1 by a selective KCa3.1 inhibitor, TRAM-34, could augment a novel interventional approach for controlling corneal fibrosis in our established in vitro model of corneal fibrosis. The expression of KCa3.1 gene and protein was analyzed in human and murine corneas. Primary human corneal fibroblast (HCF) cultures were used to examine the potential of TRAM-34 in treating corneal fibrosis by measuring levels of pro-fibrotic genes, proteins, and cellular migration using real-time quantitative qPCR, Western blotting, and scratch assay, respectively. Cytotoxicity of TRAM-34 was tested with trypan blue assay, and pro-fibrotic marker expression was tested in KCa3.1-/-. Expression of KCa3.1 mRNA and protein was detected in all three layers of the human cornea. The KCa3.1-/- mice demonstrated significantly reduced corneal fibrosis and expression of pro-fibrotic marker genes such as collagen I and α-smooth muscle actin (α-SMA), suggesting that KCa3.1 plays an important role corneal wound healing in vivo. Pharmacological treatment with TRAM-34 significantly attenuated corneal fibrosis in vitro, as demonstrated in HCFs by the inhibition TGFβ-mediated transcription of pro-fibrotic collagen I mRNA and α-SMA mRNA and protein expression (p<0.001). No evidence of cytotoxicity was observed. Our study suggests that KCa3.1 regulates corneal wound healing and that blockade of KCa3.1 by TRAM-34 offers a potential therapeutic strategy for developing therapies to cure corneal fibrosis in vivo.
Conflict of interest statement
Figures






References
-
- Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol 2012;96:614–618. doi: 10.1136/bjophthalmol-2011-300539 - DOI - PubMed
-
- Vieira-Potter VJ, Karamichos D, Lee DJ. Ocular Complications of Diabetes and Therapeutic Approaches. BioMed Res Int 2016;2016:3801570 doi: 10.1155/2016/3801570 - DOI - PMC - PubMed
-
- Ljubimov AV, Saghizadeh M. Progress in corneal wound healing. Prog Retin Eye Res 2015;49:17–45. doi: 10.1016/j.preteyeres.2015.07.002 - DOI - PMC - PubMed
-
- Wilson SE, Mohan RR, Mohan RR, Ambrosio R Jr., Hong J, Lee J. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog Retin Eye Res 2001;20:625–637. - PubMed
-
- Netto MV, Mohan RR, Ambrosio R Jr., Hutcheon AE, Zieske JD, Wilson SE. Wound healing in the cornea: a review of refractive surgery complications and new prospects for therapy. Cornea 2005;24:509–522. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

