ADPredict: ADP-ribosylation site prediction based on physicochemical and structural descriptors
- PMID: 29554239
- PMCID: PMC6061869
- DOI: 10.1093/bioinformatics/bty159
ADPredict: ADP-ribosylation site prediction based on physicochemical and structural descriptors
Abstract
Motivation: ADP-ribosylation is a post-translational modification (PTM) implicated in several crucial cellular processes, ranging from regulation of DNA repair and chromatin structure to cell metabolism and stress responses. To date, a complete understanding of ADP-ribosylation targets and their modification sites in different tissues and disease states is still lacking. Identification of ADP-ribosylation sites is required to discern the molecular mechanisms regulated by this modification. This motivated us to develop a computational tool for the prediction of ADP-ribosylated sites.
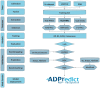
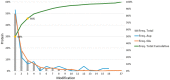
Results: Here, we present ADPredict, the first dedicated computational tool for the prediction of ADP-ribosylated aspartic and glutamic acids. This predictive algorithm is based on (i) physicochemical properties, (ii) in-house designed secondary structure-related descriptors and (iii) three-dimensional features of a set of human ADP-ribosylated proteins that have been reported in the literature. ADPredict was developed using principal component analysis and machine learning techniques; its performance was evaluated both internally via intensive bootstrapping and in predicting two external experimental datasets. It outperformed the only other available ADP-ribosylation prediction tool, ModPred. Moreover, a novel secondary structure descriptor, HM-ratio, was introduced and successfully contributed to the model development, thus representing a promising tool for bioinformatics studies, such as PTM prediction.
Availability and implementation: ADPredict is freely available at www.ADPredict.net.
Supplementary information: Supplementary data are available at Bioinformatics online.
Figures


References
-
- Bartolomei G. et al. (2016) Analysis of chromatin ADP-ribosylation at the genome-wide level and at specific loci by ADPr-ChAP. Mol. Cell, 61, 474–485. - PubMed
-
- Bilan V. et al. (2017a) Combining higher-energy collision dissociation and electron-transfer/higher-energy collision dissociation fragmentation in a product-dependent manner confidently assigns proteomewide ADP-ribose acceptor sites. Anal. Chem., 89, 1523–1530. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

