Glucose is preferentially utilized for biomass synthesis in pressure-overloaded hearts: evidence from fatty acid-binding protein-4 and -5 knockout mice
- PMID: 29554241
- PMCID: PMC6014234
- DOI: 10.1093/cvr/cvy063
Glucose is preferentially utilized for biomass synthesis in pressure-overloaded hearts: evidence from fatty acid-binding protein-4 and -5 knockout mice
Abstract
Aims: The metabolism of the failing heart is characterized by an increase in glucose uptake with reduced fatty acid (FA) oxidation. We previously found that the genetic deletion of FA-binding protein-4 and -5 [double knockout (DKO)] induces an increased myocardial reliance on glucose with decreased FA uptake in mice. However, whether this fuel switch confers functional benefit during the hypertrophic response remains open to debate. To address this question, we investigated the contractile function and metabolic profile of DKO hearts subjected to pressure overload.
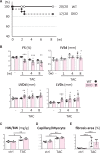
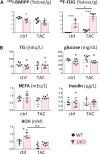
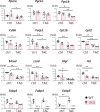
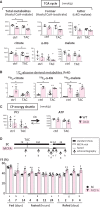
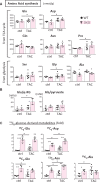
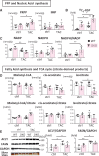
Methods and results: Transverse aortic constriction (TAC) significantly reduced cardiac contraction in DKO mice (DKO-TAC), although an increase in cardiac mass and interstitial fibrosis was comparable with wild-type TAC (WT-TAC). DKO-TAC hearts exhibited enhanced glucose uptake by 8-fold compared with WT-TAC. Metabolic profiling and isotopomer analysis revealed that the pool size in the TCA cycle and the level of phosphocreatine were significantly reduced in DKO-TAC hearts, despite a marked increase in glycolytic flux. The ingestion of a diet enriched in medium-chain FAs restored cardiac contractile dysfunction in DKO-TAC hearts. The de novo synthesis of amino acids as well as FA from glycolytic flux was unlikely to be suppressed, despite a reduction in each precursor. The pentose phosphate pathway was also facilitated, which led to the increased production of a coenzyme for lipogenesis and a precursor for nucleotide synthesis. These findings suggest that reduced FA utilization is not sufficiently compensated by a robust increase in glucose uptake when the energy demand is elevated. Glucose utilization for sustained biomass synthesis further enhances diminishment of the pool size in the TCA cycle.
Conclusions: Our data suggest that glucose is preferentially utilized for biomass synthesis rather than ATP production during pressure-overload-induced cardiac hypertrophy and that the efficient supplementation of energy substrates may restore cardiac dysfunction caused by energy insufficiency.
Figures

Comment in
-
Fatty acids are the best fuel for overloaded hearts.Cardiovasc Res. 2018 Jul 1;114(8):1055-1056. doi: 10.1093/cvr/cvy106. Cardiovasc Res. 2018. PMID: 29718157 No abstract available.
References
-
- Taegtmeyer H, Young ME, Lopaschuk GD, Abel ED, Brunengraber H, Darley-Usmar V, Des Rosiers C, Gerszten R, Glatz JF, Griffin JL, Gropler RJ, Holzhuetter HG, Kizer JR, Lewandowski ED, Malloy CR, Neubauer S, Peterson LR, Portman MA, Recchia FA, Van Eyk JE, Wang TJ.. Assessing cardiac metabolism: a scientific statement from the american heart association. Circ Res 2016;118:1659–1701. - PMC - PubMed
-
- Neubauer S. The failing heart–an engine out of fuel. N Engl J Med 2007;356:1140–1151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

