Comparing CISNET Breast Cancer Incidence and Mortality Predictions to Observed Clinical Trial Results of Mammography Screening from Ages 40 to 49
- PMID: 29554468
- PMCID: PMC5862071
- DOI: 10.1177/0272989X17718168
Comparing CISNET Breast Cancer Incidence and Mortality Predictions to Observed Clinical Trial Results of Mammography Screening from Ages 40 to 49
Abstract
Background: The UK Age trial compared annual mammography screening of women ages 40 to 49 years with no screening and found a statistically significant breast cancer mortality reduction at the 10-year follow-up but not at the 17-year follow-up. The objective of this study was to compare the observed Age trial results with the Cancer Intervention and Surveillance Modeling Network (CISNET) breast cancer model predicted results.
Methods: Five established CISNET breast cancer models used data on population demographics, screening attendance, and mammography performance from the Age trial together with extant natural history parameters to project breast cancer incidence and mortality in the control and intervention arm of the trial.
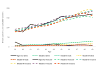
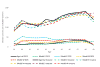
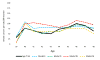
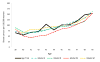
Results: The models closely reproduced the effect of annual screening from ages 40 to 49 years on breast cancer incidence. Restricted to breast cancer deaths originating from cancers diagnosed during the intervention phase, the models estimated an average 15% (range across models, 13% to 17%) breast cancer mortality reduction at the 10-year follow-up compared with 25% (95% CI, 3% to 42%) observed in the trial. At the 17-year follow-up, the models predicted 13% (range, 10% to 17%) reduction in breast cancer mortality compared with the non-significant 12% (95% CI, -4% to 26%) in the trial.
Conclusions: The models underestimated the effect of screening on breast cancer mortality at the 10-year follow-up. Overall, the models captured the observed long-term effect of screening from age 40 to 49 years on breast cancer incidence and mortality in the UK Age trial, suggesting that the model structures, input parameters, and assumptions about breast cancer natural history are reasonable for estimating the impact of screening on mortality in this age group.
Keywords: CISNET; breast cancer models; external validation; mammography trial simulation.
Figures
Similar articles
-
Annual mammographic screening to reduce breast cancer mortality in women from age 40 years: long-term follow-up of the UK Age RCT.Health Technol Assess. 2020 Oct;24(55):1-24. doi: 10.3310/hta24550. Health Technol Assess. 2020. PMID: 33141657 Free PMC article. Clinical Trial.
-
Modeling Ductal Carcinoma In Situ (DCIS): An Overview of CISNET Model Approaches.Med Decis Making. 2018 Apr;38(1_suppl):126S-139S. doi: 10.1177/0272989X17729358. Med Decis Making. 2018. PMID: 29554463 Free PMC article.
-
Comparing CISNET Breast Cancer Models Using the Maximum Clinical Incidence Reduction Methodology.Med Decis Making. 2018 Apr;38(1_suppl):112S-125S. doi: 10.1177/0272989X17743244. Med Decis Making. 2018. PMID: 29554471 Free PMC article.
-
Advanced breast cancer and breast cancer mortality in randomized controlled trials on mammography screening.J Clin Oncol. 2009 Dec 10;27(35):5919-23. doi: 10.1200/JCO.2009.22.7041. Epub 2009 Nov 2. J Clin Oncol. 2009. PMID: 19884547 Review.
-
Reflecting on 20 years of breast cancer modeling in CISNET: Recommendations for future cancer systems modeling efforts.PLoS Comput Biol. 2021 Jun 17;17(6):e1009020. doi: 10.1371/journal.pcbi.1009020. eCollection 2021 Jun. PLoS Comput Biol. 2021. PMID: 34138842 Free PMC article. Review.
Cited by
-
Personalizing Breast Cancer Screening Based on Polygenic Risk and Family History.J Natl Cancer Inst. 2021 Apr 6;113(4):434-442. doi: 10.1093/jnci/djaa127. J Natl Cancer Inst. 2021. PMID: 32853342 Free PMC article.
-
Comparing Canada's OncoSim-Breast model with the United States' Cancer Intervention and Surveillance Modeling Network (CISNET) breast cancer models.Health Rep. 2025 Jun 18;36(6):3-14. doi: 10.25318/82-003-x202500600001-eng. Health Rep. 2025. PMID: 40532081 Free PMC article.
-
Supplemental Screening as an Adjunct to Mammography for Breast Cancer Screening in People With Dense Breasts: A Health Technology Assessment.Ont Health Technol Assess Ser. 2023 Dec 19;23(9):1-293. eCollection 2023. Ont Health Technol Assess Ser. 2023. PMID: 39364436 Free PMC article.
-
Breast Cancer Screening Among Childhood Cancer Survivors Treated Without Chest Radiation: Clinical Benefits and Cost-Effectiveness.J Natl Cancer Inst. 2022 Feb 7;114(2):235-244. doi: 10.1093/jnci/djab149. J Natl Cancer Inst. 2022. PMID: 34324686 Free PMC article.
-
A Bayesian Simulation Model for Breast Cancer Screening, Incidence, Treatment, and Mortality.Med Decis Making. 2018 Apr;38(1_suppl):78S-88S. doi: 10.1177/0272989X17714473. Epub 2017 Jun 19. Med Decis Making. 2018. PMID: 28627297 Free PMC article.
References
-
- Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB, et al. Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Med Decis Making. 2012;32(5):733–43. - PubMed
-
- Plevritis S, Munoz D, Kurian A, Alagoz O, Near AM, Stout NK, et al. Contributions of screening and systemic therapy to molecular subtype specific U.S. breast cancer mortality from 2000 to 2010. 2016 submitted.
-
- Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784–92. - PubMed
-
- Siu AL On behalf of the U S. Preventive Services Task Force. Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164(4):279–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

