Simulating the Impact of Risk-Based Screening and Treatment on Breast Cancer Outcomes with MISCAN-Fadia
- PMID: 29554469
- PMCID: PMC5862065
- DOI: 10.1177/0272989X17711928
Simulating the Impact of Risk-Based Screening and Treatment on Breast Cancer Outcomes with MISCAN-Fadia
Abstract
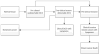
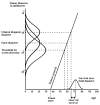
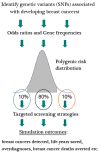
The MISCAN-Fadia microsimulation model uses continuous tumor growth to simulate the natural history of breast cancer and has been used extensively to estimate the impact of screening and adjuvant treatment on breast cancer incidence and mortality trends. The model simulates individual life histories from birth to death, with and without breast cancer, in the presence and in the absence of screening and treatment. Life histories are simulated according to discrete events such as birth, tumor inception, the tumor's clinical diagnosis diameter in the absence of screening, and death from breast cancer or death from other causes. MISCAN-Fadia consists of 4 main components: demography, natural history of breast cancer, screening, and treatment. Screening impact on the natural history of breast cancer is assessed by simulating continuous tumor growth and the "fatal diameter" concept. This concept implies that tumors diagnosed at a size that is between the screen detection threshold and the fatal diameter are cured, while tumors diagnosed at a diameter larger than the fatal tumor diameter metastasize and lead to breast cancer death. MISCAN-Fadia has been extended by including a different natural history for molecular subtypes based on a tumor's estrogen receptor (ER) status and human epidermal growth factor receptor 2 (HER2) status. In addition, personalized screening strategies that target women based on their risk such as breast density have been incorporated into the model. This personalized approach to screening will continue to develop in light of potential polygenic risk stratification possibilities and new screening modalities.
Keywords: breast cancer epidemiology; microsimulation model; risk-based breast cancer screening.
Figures
References
-
- Habbema JD, van Oortmarssen GJ, Lubbe JT, van der Maas PJ. The MISCAN simulation program for the evaluation of screening for disease. Comput Methods Programs Biomed. 1985;20(1):79–93. - PubMed
-
- Boer R, de Koning H, van Oortmarssen G, Warmerdam P, van der Maas P. Stage distribution at first and repeat examinations in breast cancer screening. J Med Screen. 1999;6(3):132–8. - PubMed
-
- Tabar L, Fagerberg G, Duffy SW, Day NE, Gad A, Grontoft O. Update of the Swedish two-county program of mammographic screening for breast cancer. Radiol Clin North Am. 1992;30(1):187–210. - PubMed
-
- Tabar L, Fagerberg G, Day NE, Duffy SW. The Swedish two-county trial of mammographic screening for breast cancer: recent results on mortality and tumor characteristics. Pathol Biol (Paris) 1992;39(9):846. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

