Comparing CISNET Breast Cancer Models Using the Maximum Clinical Incidence Reduction Methodology
- PMID: 29554471
- PMCID: PMC5862068
- DOI: 10.1177/0272989X17743244
Comparing CISNET Breast Cancer Models Using the Maximum Clinical Incidence Reduction Methodology
Abstract
Background: Collaborative modeling has been used to estimate the impact of potential cancer screening strategies worldwide. A necessary step in the interpretation of collaborative cancer screening model results is to understand how model structure and model assumptions influence cancer incidence and mortality predictions. In this study, we examined the relative contributions of the pre-clinical duration of breast cancer, the sensitivity of screening, and the improvement in prognosis associated with treatment of screen-detected cases to the breast cancer incidence and mortality predictions of 5 Cancer Intervention and Surveillance Modeling Network (CISNET) models.
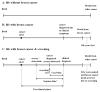
Methods: To tease out the impact of model structure and assumptions on model predictions, the Maximum Clinical Incidence Reduction (MCLIR) method compares changes in the number of breast cancers diagnosed due to clinical symptoms and cancer mortality between 4 simplified scenarios: 1) no-screening; 2) one-time perfect screening exam, which detects all existing cancers and perfect treatment (i.e., cure) of all screen-detected cancers; 3) one-time digital mammogram and perfect treatment of all screen-detected cancers; and 4) one-time digital mammogram and current guideline-concordant treatment of all screen-detected cancers.
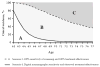
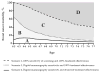
Results: The 5 models predicted a large range in maximum clinical incidence (19% to 71%) and in breast cancer mortality reduction (33% to 67%) from a one-time perfect screening test and perfect treatment. In this perfect scenario, the models with assumptions of tumor inception before it is first detectable by mammography predicted substantially higher incidence and mortality reductions than models with assumptions of tumor onset at the start of a cancer's screen-detectable phase. The range across models in breast cancer clinical incidence (11% to 24%) and mortality reduction (8% to 18%) from a one-time digital mammogram at age 62 y with observed sensitivity and current guideline-concordant treatment was considerably smaller than achievable under perfect conditions.
Conclusions: The timing of tumor inception and its effect on the length of the pre-clinical phase of breast cancer had a substantial impact on the grouping of models based on their predictions for clinical incidence and breast cancer mortality reduction. This key finding about the timing of tumor inception will be included in future CISNET breast analyses to enhance model transparency. The MCLIR approach should aid in the interpretation of variations in model results and could be adopted in other disease screening settings to enhance model transparency.
Keywords: breast cancer natural history assumptions; maximum clinical incidence reduction; screening effectiveness.
Figures

References
-
- Lee SJ, Li X, Huang H. Models for Breast Cancer Screening Strategies Updated for Ductal Carcinoma In Situ and Subgroups Medical Decision Making. 2017 In Press.
-
- van den Broek JJ, van Ravesteyn NT, Heijnsdijk EA, de Koning HJ. Estimating the effects of risk-based screening and adjuvant treatment using the MISCAN-Fadia continuous tumor growth model for breast cancer. Medical Decision Making. 2017 In Press.
-
- Huang X, Li Y, Song J, Berry DA. The MD Anderson CISNET Model for Estimating Benefits of Adjuvant Therapy and Screening Mammography for Breast Cancer: An Update. Medical Decision Making. 2017 In Press.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

