A Molecular Subtype-Specific Stochastic Simulation Model of US Breast Cancer Incidence, Survival, and Mortality Trends from 1975 to 2010
- PMID: 29554473
- PMCID: PMC6538507
- DOI: 10.1177/0272989X17737508
A Molecular Subtype-Specific Stochastic Simulation Model of US Breast Cancer Incidence, Survival, and Mortality Trends from 1975 to 2010
Abstract
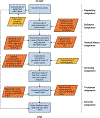
We present a Monte Carlo simulation model that reproduces US invasive breast cancer incidence and mortality trends from 1975 to 2010 as a function of screening and adjuvant treatment. This model was developed for multiple purposes, including to quantify the impact of screening and adjuvant therapy on past and current trends, predict future trends, and evaluate potential outcomes under hypothetical screening and treatment interventions. The model first generates the life histories of individual breast cancer patients by determining the patient's age, tumor size, estrogen receptor (ER) status, human epidermal growth factor 2 (HER2) status, SEER (Surveillance, Epidemiology, and End Results) historic stage, detection mode at time of detection, preclinical tumor course, and death age and cause of death (breast cancer v. other causes). The model incorporates common inputs used by the Cancer Intervention and Surveillance Modeling Network (CISNET), including the dissemination patterns for screening mammography, breast cancer survival in the absence of adjuvant therapy, dissemination and efficacy of treatment by ER and HER2 status, and death from causes other than breast cancer. In this article, predicted mortality outcomes are compared assuming proportional v. nonproportional hazards effects of treatment on breast cancer survival. We found that the proportional hazards treatment effects are sufficient for ER-negative disease. However, for ER-positive disease, the treatment effects appear to be higher during the early years following diagnosis and then diminish over time. Using nonproportional hazards effects for ER-positive cases, the predicted breast cancer mortality rates closely match the SEER mortality trends from 1975 to 2010, particularly after 1995. Our work indicates that population-level simulation modeling may have a broader role in assessing the time dependence of treatment effects.
Keywords: SEER; breast cancer incidence trends; breast cancer mortality trends; nonproportional hazards; time-dependent treatment effects.
Figures



References
-
- Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005; 353(17): 1784–1792. - PubMed
-
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005; 353(16): 1659–1672. - PubMed
-
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005; 353(16): 1673–1684. - PubMed
-
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005; 365(9472): 1687–1717. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

