Re-challenging immune checkpoint inhibitor in a patient with advanced non-small cell lung cancer: a case report
- PMID: 29554874
- PMCID: PMC5859734
- DOI: 10.1186/s12885-018-4212-1
Re-challenging immune checkpoint inhibitor in a patient with advanced non-small cell lung cancer: a case report
Abstract
Background: Currently, immune checkpoint (ICP) inhibitors are essential drugs for the treatment of non-small cell lung cancer (NSCLC). However, in patients previously treated with ICP inhibitors, the efficacy and safety of re-challenging the same or another ICP inhibitor remain unclear.
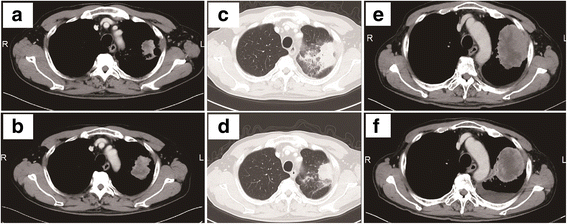
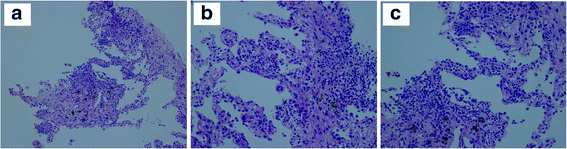
Case presentation: We present the case of a patient treated with nivolumab for advanced NSCLC who was previously treated with an ICP inhibitor as the first-line chemotherapy along with heavy cytotoxic chemotherapy. After the failure of five lines of chemotherapy, 3 cycles of nivolumab, as the ICP inhibitor re-challenge, the patient achieved a partial response.
Conclusions: This case might suggest that re-challenging an ICP inhibitor could be clinically active in selected patients with advanced NSCLC who progress after achieving an initial clinical benefit with an ICP inhibitor.
Keywords: Immune checkpoint inhibitor; Immune-related adverse events; Nivolumab; Non-small cell lung cancer; Re-challenge.
Conflict of interest statement
Ethics approval and consent to participate
The case report was waivered by the Ethics Committee of Tokyo Metropolitan Cancer and Infectious disease Center Komagome Hospital. The clinical information presented in this case report was obtained through Tokyo Metropolitan Cancer and Infectious disease Center Komagome Hospital’s medical records.
Consent for publication
Written informed consent was obtained from the patients for publication of this case report.
Competing interests
All authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. - DOI - PubMed
-
- Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

