A poly-herbal blend (Herbagut®) on adults presenting with gastrointestinal complaints: a randomised, double-blind, placebo-controlled study
- PMID: 29554961
- PMCID: PMC5859649
- DOI: 10.1186/s12906-018-2168-y
A poly-herbal blend (Herbagut®) on adults presenting with gastrointestinal complaints: a randomised, double-blind, placebo-controlled study
Abstract
Background: To evaluate the efficacy and tolerability of a poly-herbal formulation, Herbagut, for the treatment of gastrointestinal symptoms and its effect on quality of life parameters in patients presenting with self-reported, unsatisfactory bowel habits.
Methods: This was a randomised, double-blind, placebo-controlled trial. Fifty adults with self-reported unsatisfactory bowel habits, primarily characterised by chronic constipation were randomly allocated to take Herbagut or a matching placebo for 28 days. Efficacy of gastrointestinal changes was measured by the completion of a patient daily diary evaluating changes in stool type (Bristol Stool Form Scale), ease of bowel movements, and feeling of complete evacuation; and the Gastrointestinal Symptom Rating Scale (GSRS). Changes in quality of life were also examined using the World Health Organization Quality of Life - abbreviated version (WHOQOL-BREF), and the Patient Assessment of Constipation-Quality of Life (PAC-QOL).
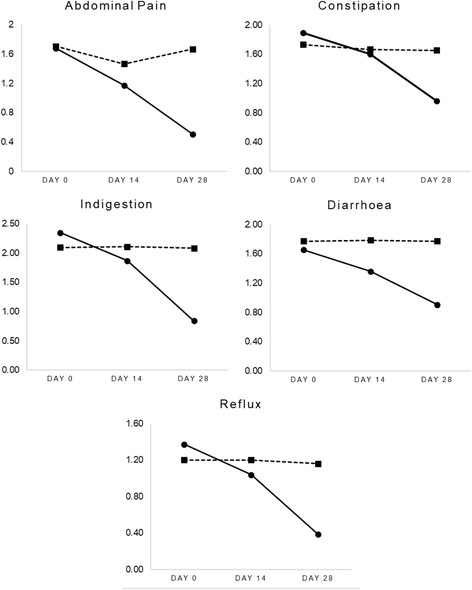
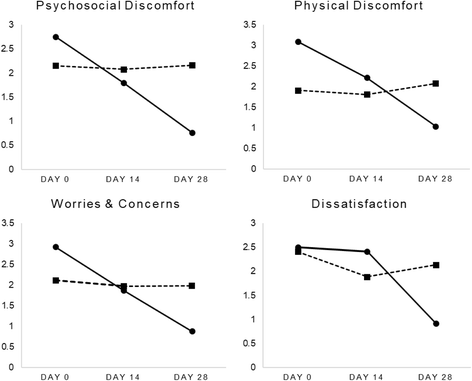
Results: All participants completed the 28-day trial with no adverse events reported. Compared to the placebo, weekly bowel movements increased over time (p < .001), as did self-reported, normal bowel motions (76% vs 4%; p < .001). Self-reported incomplete evacuation was also lower in the Herbagut group compared to placebo (24% vs 76%; p = <.001). GSRS domain ratings for abdominal pain, constipation, diarrhoea, indigestion, and reflux also decreased significantly in people taking Herbagut compared to placebo (p < .001, for all domains). Moreover, quality of life significantly improved in the Herbagut group compared to placebo as indicated by significantly greater improvement in WHOQOL-BREF domain ratings for overall quality of life, social relations, environmental health, psychological health, and physical health (p < .001, for all domains); and PAC-QOL domain ratings for physical discomfort, psychosocial discomfort, worries and concerns, and life satisfaction (p < .001, for all domains). The changes were considered clinically meaningful as evidenced by their large effect sizes.
Conclusion: Herbagut ingestion over a 28-day period resulted in improvements in several gastrointestinal symptoms and overall quality of life. Further investigation utilising larger sample sizes and diverse clinical and cultural populations are needed.
Trial registration: Clinical Trials Registry- India /2016/11/007479 . Registered 24 April 2015 (retrospectively registered).
Keywords: Clinical trial; Constipation; Digestion; Gastrointestinal function; Herbal treatment.
Conflict of interest statement
Ethics approval and consent to participate
The study protocol was approved by ‘Nagpur Independent Ethics Committee’ and all participants provided informed consent.
Consent for publication
Not applicable.
Competing interests
This study was independently managed by the principal investigator, Dr Hemant Gupta, who declares no competing interests. Dr Adrian Lopresti has received study funding from Arjuna Natural Extracts Ltd in the past for previously completed, unrelated studies. Mr Stephen Smith declares no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Lubiprostone vs Senna in postoperative orthopedic surgery patients with opioid-induced constipation: a double-blind, active-comparator trial.World J Gastroenterol. 2014 Nov 21;20(43):16323-33. doi: 10.3748/wjg.v20.i43.16323. World J Gastroenterol. 2014. PMID: 25473191 Free PMC article. Clinical Trial.
-
A Novel Herbal Composition Alleviates Functional Constipation, Reduces Gastrointestinal Transit Time, and Improves Bowel Function in Adults: A Double-Blind, Randomized Clinical Study.J Am Nutr Assoc. 2024 Aug;43(6):553-566. doi: 10.1080/27697061.2024.2346073. Epub 2024 May 1. J Am Nutr Assoc. 2024. PMID: 38691810 Clinical Trial.
-
The effect of 'Zesy002' kiwifruit (Actinidia chinensis var. chinensis) on gut health function: a randomised cross-over clinical trial.J Nutr Sci. 2019 May 3;8:e18. doi: 10.1017/jns.2019.14. eCollection 2019. J Nutr Sci. 2019. PMID: 31080591 Free PMC article. Clinical Trial.
-
Range and consistency of gastrointestinal outcomes reported in peritoneal dialysis trials: A systematic review.Perit Dial Int. 2023 Jul;43(4):315-323. doi: 10.1177/08968608221126849. Epub 2022 Sep 20. Perit Dial Int. 2023. PMID: 36127835
-
Holistic Approach to Functional Constipation: Perspective of Traditional Persian Medicine.Chin J Integr Med. 2019 Nov;25(11):867-872. doi: 10.1007/s11655-015-2302-3. Epub 2015 Nov 23. Chin J Integr Med. 2019. PMID: 26597285 Review.
Cited by
-
Efficacy of a curcumin extract (Curcugen™) on gastrointestinal symptoms and intestinal microbiota in adults with self-reported digestive complaints: a randomised, double-blind, placebo-controlled study.BMC Complement Med Ther. 2021 Jan 21;21(1):40. doi: 10.1186/s12906-021-03220-6. BMC Complement Med Ther. 2021. PMID: 33478482 Free PMC article. Clinical Trial.
-
Non-Chinese herbal medicines for functional dyspepsia.Cochrane Database Syst Rev. 2023 Jun 15;6(6):CD013323. doi: 10.1002/14651858.CD013323.pub2. Cochrane Database Syst Rev. 2023. PMID: 37323050 Free PMC article.
References
-
- Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology. 150(6):1262–279. - PubMed
-
- Addolorato G, Mirijello A, D'Angelo C, Leggio L, Ferrulli A, Abenavoli L, Vonghia L, Cardone S, Leso V, Cossari A, et al. State and trait anxiety and depression in patients affected by gastrointestinal diseases: psychometric evaluation of 1641 patients referred to an internal medicine outpatient setting. Int J Clin Pract. 2008;62(7):1063–1069. doi: 10.1111/j.1742-1241.2008.01763.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous