CHF-PROM: validation of a patient-reported outcome measure for patients with chronic heart failure
- PMID: 29554963
- PMCID: PMC5859646
- DOI: 10.1186/s12955-018-0874-2
CHF-PROM: validation of a patient-reported outcome measure for patients with chronic heart failure
Abstract
Background: Due to a lack of an appropriate disease-specific patient-reported outcome (PRO) instrument for chronic heart failure including its social support and treatment aspects in China, this study was performed to develop a patient-reported outcome measure (PROM) for patients with chronic heart failure and evaluate its reliability, validity, and feasibility.
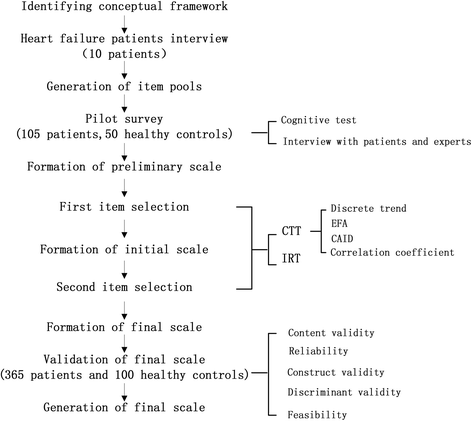
Methods: According to the standard PROM guidelines established by the Food and Drug Administration, an item pool was formed by reviewing a large amount of relevant literature and interviewing patients with chronic heart failure about their main symptoms. Thus, the primary scale was created after adjusting the items and language with the help of patients and experts in the field. Next, 155 patients from 8 hospitals in different districts were recruited for a pilot survey using questionnaires containing these items. The patients' responses were analyzed using the classical test theory and item response theory to select high-quality items and determine the subdomains of the scale. This was followed by a formal investigation in the same eight hospitals. In total, 360 patients and 100 healthy subjects were included to evaluate the reliability, validity, and feasibility of the items. Through this process, the final scale was established.
Results: The final scale comprised 12 subdomains with 57 items related to physical, psychological, social, and therapeutic areas. The data analysis results of the formal investigation showed that the PROM for chronic heart failure had good reliability, validity, and feasibility. Reliability was verified by Cronbach's alpha coefficient, which was 0.913 for the total scale, 0.903 for the physical domain, 0.941 for the psychological domain, 0.827 for the social domain, and 0.839 for the therapeutic domain. The construct validity results met the relative criteria of confirmatory factor analysis. Discriminant validity was represented by score comparisons of nine subdomains. The response rate and the effective rate of return of the CHF-PROM were 98.94% and 98.92%, respectively.
Conclusions: The final scale coincides with the theoretical framework and better reflects the overall quality of life of patients with chronic heart failure. This scale can be used as a valid instrument to evaluate clinical treatment and clinical trials of chronic heart failure.
Keywords: Chronic heart failure (CHF); Item response theory (IRT); Item selection; Patient-reported outcome (PRO); Reliability; Validity.
Conflict of interest statement
Ethics approval and consent to participate
The study protocol received medical and ethical approval from Shanxi Medical University. All participants provided written informed consent and received compensation for their time and effort.
Consent for publication
All authors have approved the manuscript for publication.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Mariell Jessup, Biykem Bozkurt, Javed Butler, et al. 2013 ACCF/AHA guideline for the Management of Heart Failure. JACC 2013; 62:147–239. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous