Comparison of Diagnostic Methods and Sampling Sites for the Detection of Demodex musculi
- PMID: 29555007
- PMCID: PMC5868384
Comparison of Diagnostic Methods and Sampling Sites for the Detection of Demodex musculi
Abstract
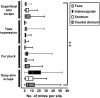
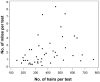
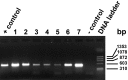
Demodex mites are microscopic, cigar-shaped, follicular mites often regarded as commensal microfauna in mammals. Although Demodex spp. can cause dermatologic disease in any immunocompromised mammal, they are rarely reported in laboratory mice. Recent identification of Demodex musculi in a colony of immunodeficient mice with dermatitis afforded us the opportunity to investigate the comparative sensitivity of 4 antemortem diagnostic techniques to detect D. musculi-superficial skin scrape (SSS), tape impression (TI), fur pluck (FP), and deep skin scrape (DSS)-which we performed on 4 anatomic sites (face, interscapular region [IS], caudal ventrum [CV], and caudal dorsum [CD]) in 46 mice. DSS had an overall detection rate of 91.1% (n = 112 tests), with the highest detection rates in IS (93.5%), CV (89.1%), and CD (90.0%). The detection rates for SSS (62.5%; n = 112 tests), TI (57.5%; n = 138 tests), and FP (62.7%; n = 158 tests) were all lower than for DSS. IS was the most reliable site. Results from combined FP and DSS samples collected from IS and CV yielded 100% detection, whereas the face was not a desirable sampling site due to inadequate sample quality and low detection rate. Demodex eggs and larvae were observed from FP more often than DSS (19.0% of 158 tests compared with 14.3% of 112 tests). In a subset of samples, an 18S rRNA PCR assay was equivalent to DSS for detection of mites (both 100%, n = 8). We recommend collecting samples from both IS and CV by both FP and DSS to assess for the presence of D. musculi and performing further studies to assess whether PCR analysis can be used as a diagnostic tool for the detection of Demodex mites in laboratory mice.
Figures


Similar articles
-
Ivermectin-compounded Feed Compared with Topical Moxidectin-Imidacloprid for Eradication of Demodex musculi in Laboratory Mice.J Am Assoc Lab Anim Sci. 2018 Sep 1;57(5):483-497. doi: 10.30802/AALAS-JAALAS-18-000003. Epub 2018 Sep 5. J Am Assoc Lab Anim Sci. 2018. PMID: 30185284 Free PMC article.
-
Detection of Myocoptes musculinus in Fur Swab and Fecal Samples by Using PCR Analysis.J Am Assoc Lab Anim Sci. 2019 Nov 1;58(6):796-801. doi: 10.30802/AALAS-JAALAS-19-000046. Epub 2019 Oct 29. J Am Assoc Lab Anim Sci. 2019. PMID: 31662161 Free PMC article.
-
Characterization of Demodex musculi Infestation, Associated Comorbidities, and Topographic Distribution in a Mouse Strain with Defective Adaptive Immunity.Comp Med. 2017 Aug 1;67(4):315-329. Comp Med. 2017. PMID: 28830578 Free PMC article.
-
Demodex folliculorum and Demodex brevis as a cause of chronic marginal blepharitis.Ann Acad Med Stetin. 2007;53(1):63-7; discussion 67. Ann Acad Med Stetin. 2007. PMID: 18561612 Review.
-
Demodex mites.Clin Dermatol. 2014 Nov-Dec;32(6):739-43. doi: 10.1016/j.clindermatol.2014.02.012. Epub 2014 Feb 28. Clin Dermatol. 2014. PMID: 25441466 Review.
Cited by
-
Demodicosis in Different Age Groups and Alternative Treatment Options-A Review.J Clin Med. 2023 Feb 19;12(4):1649. doi: 10.3390/jcm12041649. J Clin Med. 2023. PMID: 36836184 Free PMC article. Review.
-
Detection and Eradication of a Demodex Infestation in Specific Pathogen-free High-barrier Laboratory Mouse Facility Housing Immunocompromised Animals.J Am Assoc Lab Anim Sci. 2024 Jun 22;63(5):521-9. doi: 10.30802/AALAS-JAALAS-23-000092. Online ahead of print. J Am Assoc Lab Anim Sci. 2024. PMID: 38908907 Free PMC article.
-
Ivermectin-compounded Feed Compared with Topical Moxidectin-Imidacloprid for Eradication of Demodex musculi in Laboratory Mice.J Am Assoc Lab Anim Sci. 2018 Sep 1;57(5):483-497. doi: 10.30802/AALAS-JAALAS-18-000003. Epub 2018 Sep 5. J Am Assoc Lab Anim Sci. 2018. PMID: 30185284 Free PMC article.
-
Detection of Myocoptes musculinus in Fur Swab and Fecal Samples by Using PCR Analysis.J Am Assoc Lab Anim Sci. 2019 Nov 1;58(6):796-801. doi: 10.30802/AALAS-JAALAS-19-000046. Epub 2019 Oct 29. J Am Assoc Lab Anim Sci. 2019. PMID: 31662161 Free PMC article.
-
Epidemiology of sarcoptic mange in free-ranging vicuñas (Vicugna vicugna): a cross-sectional study in Andean highland communities in Peru.Rev Bras Parasitol Vet. 2024 Jul 8;33(2):e020523. doi: 10.1590/S1984-29612024030. eCollection 2024. Rev Bras Parasitol Vet. 2024. PMID: 38985054 Free PMC article.
References
-
- Akilov OE, Kazanceva SV, Vlasova IA. 2001. Particular features of immune response after invasion of different species of human Demodex mites. Russ J Immunol 6:399–404. - PubMed
-
- Akilov OE, Mumcuoglu KY. 2004. Immune response in demodicosis. J Eur Acad Dermatol Venereol 18:440–444. - PubMed
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. - PubMed
-
- Barthold SW, Griffey SM, Percy DH. 2016. Pathology of laboratory rodents and rabbits, 4th ed Ames (IA): Blackwell Publishing.
-
- Bourdeau PJ. 2010. Variation of size in Demodex canis: from the shortest to the longest forms. The 24th Annual Congress of the ECVD-ESVD, 17–19 September 2009, Bled, Slovenia. Vet Dermatol 21:213.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
