Anticipatory prefrontal cortex activity underlies stress-induced changes in Pavlovian fear conditioning
- PMID: 29555429
- PMCID: PMC5949265
- DOI: 10.1016/j.neuroimage.2018.03.030
Anticipatory prefrontal cortex activity underlies stress-induced changes in Pavlovian fear conditioning
Abstract
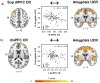
Excessive stress exposure often leads to emotional dysfunction, characterized by disruptions in healthy emotional learning, expression, and regulation processes. A prefrontal cortex (PFC)-amygdala circuit appears to underlie these important emotional processes. However, limited human neuroimaging research has investigated whether these brain regions underlie the altered emotional function that develops with stress. Therefore, the present study used functional magnetic resonance imaging (fMRI) to investigate stress-induced changes in PFC-amygdala function during Pavlovian fear conditioning. Participants completed a variant of the Montreal Imaging Stress Task (MIST) followed (25 min later) by a Pavlovian fear conditioning task during fMRI. Self-reported stress to the MIST was used to identify three stress-reactivity groups (Low, Medium, and High). Psychophysiological, behavioral, and fMRI signal responses were compared between the three stress-reactivity groups during fear conditioning. Fear learning, indexed via participant expectation of the unconditioned stimulus during conditioning, increased with stress reactivity. Further, the High stress-reactivity group demonstrated greater autonomic arousal (i.e., skin conductance response, SCR) to both conditioned and unconditioned stimuli compared to the Low and Medium stress-reactivity groups. Finally, the High stress group did not regulate the emotional response to threat. More specifically, the High stress-reactivity group did not show a negative relationship between conditioned and unconditioned SCRs. Stress-induced changes in these emotional processes paralleled changes in dorsolateral, dorsomedial, and ventromedial PFC function. These findings demonstrate that acute stress facilitates fear learning, enhances autonomic arousal, and impairs emotion regulation, and suggests these stress-induced changes in emotional function are mediated by the PFC.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures






Similar articles
-
Neural mechanisms of human temporal fear conditioning.Neurobiol Learn Mem. 2016 Dec;136:97-104. doi: 10.1016/j.nlm.2016.09.019. Epub 2016 Sep 28. Neurobiol Learn Mem. 2016. PMID: 27693343 Free PMC article.
-
Controllability modulates the neural response to predictable but not unpredictable threat in humans.Neuroimage. 2015 Oct 1;119:371-81. doi: 10.1016/j.neuroimage.2015.06.086. Epub 2015 Jul 3. Neuroimage. 2015. PMID: 26149610 Free PMC article.
-
Affective state and locus of control modulate the neural response to threat.Neuroimage. 2015 Nov 1;121:217-26. doi: 10.1016/j.neuroimage.2015.07.034. Epub 2015 Jul 18. Neuroimage. 2015. PMID: 26196669 Free PMC article.
-
Pavlovian conditioned diminution of the neurobehavioral response to threat.Neurosci Biobehav Rev. 2018 Jan;84:218-224. doi: 10.1016/j.neubiorev.2017.11.021. Epub 2017 Dec 2. Neurosci Biobehav Rev. 2018. PMID: 29203422 Free PMC article. Review.
-
The amygdala and medial prefrontal cortex: partners in the fear circuit.J Physiol. 2013 May 15;591(10):2381-91. doi: 10.1113/jphysiol.2012.248575. Epub 2013 Feb 18. J Physiol. 2013. PMID: 23420655 Free PMC article. Review.
Cited by
-
Trauma exposure acutely alters neural function during Pavlovian fear conditioning.Cortex. 2018 Dec;109:1-13. doi: 10.1016/j.cortex.2018.08.015. Epub 2018 Sep 1. Cortex. 2018. PMID: 30265859 Free PMC article.
-
Stress-Induced Changes in Effective Connectivity During Regulation of the Emotional Response to Threat.Brain Connect. 2022 Sep;12(7):629-638. doi: 10.1089/brain.2021.0062. Epub 2021 Dec 31. Brain Connect. 2022. PMID: 34541896 Free PMC article.
-
Repeatability of Neural and Autonomic Responses to Acute Psychosocial Stress.Front Neurosci. 2020 Nov 27;14:585509. doi: 10.3389/fnins.2020.585509. eCollection 2020. Front Neurosci. 2020. PMID: 33328855 Free PMC article.
-
Stress-elicited neural activity in young adults varies with childhood sexual abuse.Cortex. 2021 Apr;137:108-123. doi: 10.1016/j.cortex.2020.12.020. Epub 2021 Jan 25. Cortex. 2021. PMID: 33609897 Free PMC article.
-
Negative life experiences contribute to racial differences in the neural response to threat.Neuroimage. 2019 Nov 15;202:116086. doi: 10.1016/j.neuroimage.2019.116086. Epub 2019 Aug 8. Neuroimage. 2019. PMID: 31401241 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

