Effects of tunable, 3D-bioprinted hydrogels on human brown adipocyte behavior and metabolic function
- PMID: 29555462
- PMCID: PMC6066177
- DOI: 10.1016/j.actbio.2018.03.021
Effects of tunable, 3D-bioprinted hydrogels on human brown adipocyte behavior and metabolic function
Abstract
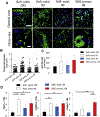
Obesity and its related health complications cause billions of dollars in healthcare costs annually in the United States, and there are yet to be safe and long-lasting anti-obesity approaches. Using brown adipose tissue (BAT) is a promising approach, as it uses fats for energy expenditure. However, the effect of the microenvironment on human thermogenic brown adipogenesis and how to generate clinically relevant sized and functioning BAT are still unknown. In our current study, we evaluated the effects of endothelial growth medium exposure on brown adipogenesis of human brown adipose progenitors (BAP). We found that pre-exposing BAP to angiogenic factors promoted brown adipogenic differentiation and metabolic activity. We further 3D bioprinted brown and white adipose progenitors within hydrogel-based bioink with controllable physicochemical properties and evaluated the cell responses in 3D bioprinted environments. We used soft, stiff, and stiff-porous constructs to encapsulate the cells. All three types had high cell viability and allowed for varying levels of function for both white and brown adipocytes. We found that the soft hydrogel constructs promoted white adipogenesis, while the stiff-porous hydrogel constructs improved both white and brown adipogenesis and were the optimal condition for promoting brown adipogenesis. Consistently, stiff-porous hydrogel constructs showed higher metabolic activities than stiff hydrogel constructs, as assessed by 2-deoxy glucose uptake (2-DOG) and oxygen consumption rate (OCR). These findings show that the physicochemical environments affect the brown adipogenesis and metabolic function, and further tuning will be able to optimize their functions. Our results also demonstrate that 3D bioprinting of brown adipose tissues with clinically relevant size and metabolic activity has the potential to be a viable option in the treatment of obesity and type 2 diabetes.
Statement of significance: One promising strategy for the treatment or prevention of obesity-mediated health complications is augmenting brown adipose tissues (BAT), which is a specialized fat that actively dissipate energy in the form of heat and maintain energy balance. In this study, we determined how pre-exposing human brown adipose progenitors (BAP) to angiogenic factors in 2D and how bioprinted microenvironments in 3D affected brown adipogenic differentiation and metabolic activity. We demonstrated that white and brown adipogenesis, and thermogenesis were regulated by tuning the bioprintable matrix stiffness and construct structure. This study not only unveils the interaction between BAP and 3D physiological microenvironments, but also presents a novel tissue engineered strategy to manage obesity and other related metabolic disorders.
Keywords: Brown adipocytes; Obesity; Porosity; Stiffness; Tissue engineering.
Copyright © 2018 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures



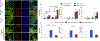

Similar articles
-
Opportunities and challenges in three-dimensional brown adipogenesis of stem cells.Biotechnol Adv. 2015 Nov 1;33(6 Pt 1):962-79. doi: 10.1016/j.biotechadv.2015.07.005. Epub 2015 Jul 29. Biotechnol Adv. 2015. PMID: 26231586 Free PMC article. Review.
-
Mechanically robust cryogels with injectability and bioprinting supportability for adipose tissue engineering.Acta Biomater. 2018 Jul 1;74:131-142. doi: 10.1016/j.actbio.2018.05.044. Epub 2018 May 26. Acta Biomater. 2018. PMID: 29842971
-
miR-199a-3p regulates brown adipocyte differentiation through mTOR signaling pathway.Mol Cell Endocrinol. 2018 Nov 15;476:155-164. doi: 10.1016/j.mce.2018.05.005. Epub 2018 Jun 28. Mol Cell Endocrinol. 2018. PMID: 29753771
-
White, brown, beige/brite: different adipose cells for different functions?Endocrinology. 2013 Sep;154(9):2992-3000. doi: 10.1210/en.2013-1403. Epub 2013 Jun 19. Endocrinology. 2013. PMID: 23782940 Review.
-
RGS2: A multifunctional signaling hub that balances brown adipose tissue function and differentiation.Mol Metab. 2019 Dec;30:173-183. doi: 10.1016/j.molmet.2019.09.015. Epub 2019 Oct 5. Mol Metab. 2019. PMID: 31767169 Free PMC article.
Cited by
-
Photocrosslinkable Biomaterials for 3D Bioprinting: Mechanisms, Recent Advances, and Future Prospects.Int J Mol Sci. 2024 Nov 22;25(23):12567. doi: 10.3390/ijms252312567. Int J Mol Sci. 2024. PMID: 39684279 Free PMC article. Review.
-
Brown Adipocyte and Splenocyte Co-Culture Maintains Regulatory T Cell Subset in Intermittent Hypobaric Conditions.Tissue Eng Regen Med. 2019 Aug 19;16(5):539-548. doi: 10.1007/s13770-019-00205-y. eCollection 2019 Oct. Tissue Eng Regen Med. 2019. PMID: 31624708 Free PMC article.
-
In vitro tissue-engineered adipose constructs for modeling disease.BMC Biomed Eng. 2019;1:27. doi: 10.1186/s42490-019-0027-7. Epub 2019 Oct 29. BMC Biomed Eng. 2019. PMID: 32133436 Free PMC article.
-
Human Adipose Derived Cells in Two- and Three-Dimensional Cultures: Functional Validation of an In Vitro Fat Construct.Stem Cells Int. 2020 Jun 10;2020:4242130. doi: 10.1155/2020/4242130. eCollection 2020. Stem Cells Int. 2020. PMID: 32587620 Free PMC article.
-
Studying Brown Adipose Tissue in a Human in vitro Context.Front Endocrinol (Lausanne). 2020 Sep 15;11:629. doi: 10.3389/fendo.2020.00629. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33042008 Free PMC article. Review.
References
-
- Wong D, Sullivan K, Heap G, The pharmaceutical market for obesity therapies, Nat. Rev. Drug Discovery 11 (2012) 669–670. - PubMed
-
- Lidell ME, Betz MJ, Leinhard OD, Heglind M, Elander L, Slawik M, Mussack T, Nilsson D, Romu T, Nuutila P, Virtanen KA, Beuschlein F, Persson A, Borga M, Enerbäck S, Evidence for two types of brown adipose tissue in humans, Nat. Med 19 (2013) 631–634. - PubMed
-
- Chondronikola M, Volpi E, Børsheim E, Porter C, Annamalai P, Enerbäck S, Lidell ME, Saraf MK, Labbe SM, Hurren NM, Yfanti C, Chao T, Andersen CR, Cesani FI, Hawkins H, Sidossis LS, Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans, Diabetes 63 (2014) 4089–4099. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

