Aberrant fatty acid metabolism in skeletal muscle contributes to insulin resistance in zinc transporter 7 (znt7)-knockout mice
- PMID: 29555680
- PMCID: PMC5961028
- DOI: 10.1074/jbc.M117.817692
Aberrant fatty acid metabolism in skeletal muscle contributes to insulin resistance in zinc transporter 7 (znt7)-knockout mice
Abstract
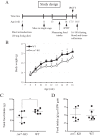
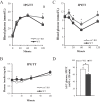
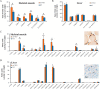
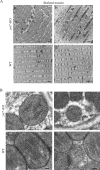
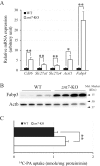
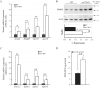
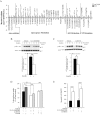
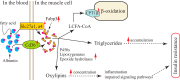
ZnT7 (Slc30a7) is a widely expressed zinc transporter involved in sequestration of zinc into the Golgi apparatus and vesicular compartments. znt7-knockout (KO) mice are mildly zinc-deficient and lean. Despite their lean phenotype, adult male znt7-KO mice are prone to insulin resistance. We hypothesized that fat partitioning from adipose to nonadipose tissues causes insulin resistance in znt7-KO mice. Here, we used biological and biochemical methods, including fatty acid and oxylipin profiling, EM, immunohistochemistry, quantitative RT-PCR, and Western blot analysis, to identify the underlying mechanism of insulin resistance in znt7-KO mice. We found that insulin resistance in this model was primarily associated with increased intracellular fatty acid levels in the skeletal muscle, which promoted intracellular lipid accumulation and production of bioactive lipid mediators, such as 12,13-dihydroxyoctadecanoic acid (12,13-DiHOME) and 12-hydroxyeicosatetraenoic acid (12-HETE). The expression of fatty acid-binding protein 3 (Fabp3) was dramatically up-regulated in the znt7-KO muscle cells accompanied by increased expression of Cd36, Slc27a1, and Slc27a4, the three major fatty acid transporters in the skeletal muscle. We also demonstrated that znt7-KO muscle cells had increased fatty acid oxidative capacity, indicated by enlarged mitochondria and increased mRNA or protein expression of key enzymes involved in the fatty acid mitochondrial shuttle and β-oxidation. We conclude that increased fatty acid uptake in the znt7-KO skeletal muscle is a key factor that contributes to the excessive intracellular lipid deposit and elevated production of bioactive lipid mediators. These mediators may play pivotal roles in oxidative stress and inflammation, leading to insulin resistance.
Keywords: Slc30a7; ZnT7; fatty acid metabolism; glucose metabolism; impaired glucose metabolism; insulin resistance; long-chain fatty acid; skeletal muscle; transporter; triglyceride; zinc; zinc transporter 7.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article. USDA is an equal opportunity provider and employer
Figures

Similar articles
-
Znt7-null mice are more susceptible to diet-induced glucose intolerance and insulin resistance.J Biol Chem. 2012 Sep 28;287(40):33883-96. doi: 10.1074/jbc.M111.309666. Epub 2012 Aug 1. J Biol Chem. 2012. PMID: 22854958 Free PMC article.
-
Zinc transporter 7 deficiency affects lipid synthesis in adipocytes by inhibiting insulin-dependent Akt activation and glucose uptake.FEBS J. 2016 Jan;283(2):378-94. doi: 10.1111/febs.13582. Epub 2015 Dec 12. FEBS J. 2016. PMID: 26524605
-
Extremely rapid increase in fatty acid transport and intramyocellular lipid accumulation but markedly delayed insulin resistance after high fat feeding in rats.Diabetologia. 2015 Oct;58(10):2381-91. doi: 10.1007/s00125-015-3691-8. Epub 2015 Jul 22. Diabetologia. 2015. PMID: 26197708
-
Role of fatty acid uptake and fatty acid beta-oxidation in mediating insulin resistance in heart and skeletal muscle.Biochim Biophys Acta. 2010 Jan;1801(1):1-22. doi: 10.1016/j.bbalip.2009.09.014. Epub 2009 Sep 24. Biochim Biophys Acta. 2010. PMID: 19782765 Review.
-
Cytosolic lipid excess-induced mitochondrial dysfunction is the cause or effect of high fat diet-induced skeletal muscle insulin resistance: a molecular insight.Mol Biol Rep. 2019 Feb;46(1):957-963. doi: 10.1007/s11033-018-4551-7. Epub 2018 Dec 8. Mol Biol Rep. 2019. PMID: 30535784 Review.
Cited by
-
Association between the Thigh Muscle and Insulin Resistance According to Body Mass Index in Middle-Aged Korean Adults.Diabetes Metab J. 2020 Jun;44(3):446-457. doi: 10.4093/dmj.2019.0110. Epub 2020 Apr 16. Diabetes Metab J. 2020. PMID: 32431102 Free PMC article.
-
Zinc.Adv Food Nutr Res. 2021;96:251-310. doi: 10.1016/bs.afnr.2021.01.003. Epub 2021 May 24. Adv Food Nutr Res. 2021. PMID: 34112355 Free PMC article. Review.
-
Roles of ZnT86D in Neurodevelopment and Pathogenesis of Alzheimer Disease in a Drosophila melanogaster Model.Int J Mol Sci. 2022 Oct 5;23(19):11832. doi: 10.3390/ijms231911832. Int J Mol Sci. 2022. PMID: 36233134 Free PMC article.
-
A novel phenolic formulation for treating hepatic and peripheral insulin resistance by regulating GLUT4-mediated glucose uptake.J Tradit Complement Med. 2021 Aug 8;12(2):195-205. doi: 10.1016/j.jtcme.2021.08.004. eCollection 2022 Mar. J Tradit Complement Med. 2021. PMID: 35528476 Free PMC article.
-
Maintenance of Intestinal Epithelial Homeostasis by Zinc Transporters.Dig Dis Sci. 2019 Sep;64(9):2404-2415. doi: 10.1007/s10620-019-05561-2. Epub 2019 Mar 4. Dig Dis Sci. 2019. PMID: 30830525 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

