A Quorum Sensing-Regulated Protein Binds Cell Wall Components and Enhances Lysozyme Resistance in Streptococcus pyogenes
- PMID: 29555699
- PMCID: PMC5952397
- DOI: 10.1128/JB.00701-17
A Quorum Sensing-Regulated Protein Binds Cell Wall Components and Enhances Lysozyme Resistance in Streptococcus pyogenes
Abstract
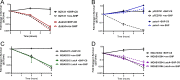
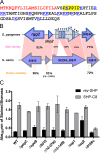
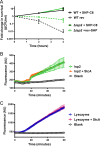
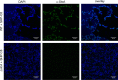
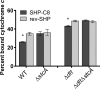
The Rgg2/3 quorum sensing (QS) system is conserved among all sequenced isolates of group A Streptococcus (GAS; Streptococcus pyogenes). The molecular architecture of the system consists of a transcriptional activator (Rgg2) and a transcriptional repressor (Rgg3) under the control of autoinducing peptide pheromones (SHP2 and SHP3). Activation of the Rgg2/3 pathway leads to increases in biofilm formation and resistance to the bactericidal effects of the host factor lysozyme. In this work, we show that deletion of a small gene, spy49_0414c, abolished both phenotypes in response to pheromone signaling. The gene encodes a small, positively charged, secreted protein, referred to as StcA. Analysis of recombinant StcA showed that it can directly interact with GAS cell wall preparations containing phosphodiester-linked carbohydrate polymers but not with preparations devoid of them. Immunofluorescence microscopy detected antibody against StcA bound to the surface of paraformaldehyde-fixed wild-type cells. Expression of StcA in bacterial culture induced a shift in the electrostatic potential of the bacterial cell surface, which became more positively charged. These results suggest that StcA promotes phenotypes by way of ionic interactions with the GAS cell wall, most likely with negatively charged cell wall-associated polysaccharides.IMPORTANCE This study focuses on a small protein, StcA, that is expressed and secreted under induction of Rgg2/3 QS, ionically associating with negatively charged domains on the cell surface. These data present a novel mechanism of resistance to the host factor lysozyme by GAS and have implications in the relevance of this circuit in the interaction between the bacterium and the human host that is mediated by the bacterial cell surface.
Keywords: CHAP domain; S-layers; biofilms; cationic peptides; murein hydrolases; pheromone; surface protein; wall polysaccharides.
Copyright © 2018 American Society for Microbiology.
Figures

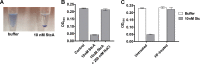
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

