Retinal isomerization and water-pore formation in channelrhodopsin-2
- PMID: 29555736
- PMCID: PMC5889620
- DOI: 10.1073/pnas.1700091115
Retinal isomerization and water-pore formation in channelrhodopsin-2
Abstract
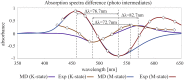
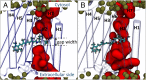
Channelrhodopsin-2 (ChR2) is a light-sensitive ion channel widely used in optogenetics. Photoactivation triggers a trans-to-cis isomerization of a covalently bound retinal. Ensuing conformational changes open a cation-selective channel. We explore the structural dynamics in the early photocycle leading to channel opening by classical (MM) and quantum mechanical (QM) molecular simulations. With QM/MM simulations, we generated a protein-adapted force field for the retinal chromophore, which we validated against absorption spectra. In a 4-µs MM simulation of a dark-adapted ChR2 dimer, water entered the vestibules of the closed channel. Retinal all-trans to 13-cis isomerization, simulated with metadynamics, triggered a major restructuring of the charge cluster forming the channel gate. On a microsecond time scale, water penetrated the gate to form a membrane-spanning preopen pore between helices H1, H2, H3, and H7. This influx of water into an ion-impermeable preopen pore is consistent with time-resolved infrared spectroscopy and electrophysiology experiments. In the retinal 13-cis state, D253 emerged as the proton acceptor of the Schiff base. Upon proton transfer from the Schiff base to D253, modeled by QM/MM simulations, we obtained an early-M/P2390-like intermediate. Rapid rotation of the unprotonated Schiff base toward the cytosolic side effectively prevents its reprotonation from the extracellular side. From MM and QM simulations, we gained detailed insight into the mechanism of ChR2 photoactivation and early events in pore formation. By rearranging the network of charges and hydrogen bonds forming the gate, water emerges as a key player in light-driven ChR2 channel opening.
Keywords: ChR2; QM/MM protein modeling; channelrhodopsin-2; molecular dynamics; optogenetics.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

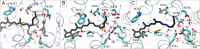

Similar articles
-
Formation Mechanism of Ion Channel in Channelrhodopsin-2: Molecular Dynamics Simulation and Steering Molecular Dynamics Simulations.Int J Mol Sci. 2019 Aug 2;20(15):3780. doi: 10.3390/ijms20153780. Int J Mol Sci. 2019. PMID: 31382458 Free PMC article.
-
Unifying photocycle model for light adaptation and temporal evolution of cation conductance in channelrhodopsin-2.Proc Natl Acad Sci U S A. 2019 May 7;116(19):9380-9389. doi: 10.1073/pnas.1818707116. Epub 2019 Apr 19. Proc Natl Acad Sci U S A. 2019. PMID: 31004059 Free PMC article.
-
The Desensitized Channelrhodopsin-2 Photointermediate Contains 13 -cis, 15 -syn Retinal Schiff Base.Angew Chem Int Ed Engl. 2021 Jul 19;60(30):16442-16447. doi: 10.1002/anie.202015797. Epub 2021 Jun 17. Angew Chem Int Ed Engl. 2021. PMID: 33973334 Free PMC article.
-
Channelrhodopsin unchained: structure and mechanism of a light-gated cation channel.Biochim Biophys Acta. 2014 May;1837(5):626-42. doi: 10.1016/j.bbabio.2013.10.014. Epub 2013 Nov 7. Biochim Biophys Acta. 2014. PMID: 24212055 Review.
-
Channelrhodopsins: a bioinformatics perspective.Biochim Biophys Acta. 2014 May;1837(5):643-55. doi: 10.1016/j.bbabio.2013.11.005. Epub 2013 Nov 16. Biochim Biophys Acta. 2014. PMID: 24252597 Review.
Cited by
-
An Atomistic Model of a Precursor State of Light-Induced Channel Opening of Channelrhodopsin.Biophys J. 2018 Oct 2;115(7):1281-1291. doi: 10.1016/j.bpj.2018.08.024. Epub 2018 Aug 27. Biophys J. 2018. PMID: 30236783 Free PMC article.
-
The effect on ion channel of different protonation states of E90 in channelrhodopsin-2: a molecular dynamics simulation.RSC Adv. 2021 Apr 19;11(24):14542-14551. doi: 10.1039/d1ra01879e. eCollection 2021 Apr 15. RSC Adv. 2021. PMID: 35424009 Free PMC article.
-
Metadynamics simulations reveal mechanisms of Na+ and Ca2+ transport in two open states of the channelrhodopsin chimera, C1C2.PLoS One. 2024 Sep 6;19(9):e0309553. doi: 10.1371/journal.pone.0309553. eCollection 2024. PLoS One. 2024. PMID: 39241014 Free PMC article.
-
Channelrhodopsin C1C2: Photocycle kinetics and interactions near the central gate.Biophys J. 2021 May 4;120(9):1835-1845. doi: 10.1016/j.bpj.2021.03.002. Epub 2021 Mar 9. Biophys J. 2021. PMID: 33705762 Free PMC article.
-
The Mechanism of the Channel Opening in Channelrhodopsin-2: A Molecular Dynamics Simulation.Int J Mol Sci. 2023 Mar 16;24(6):5667. doi: 10.3390/ijms24065667. Int J Mol Sci. 2023. PMID: 36982741 Free PMC article.
References
-
- Nagel G, et al. Channelrhodopsin-1: a light-gated proton channel in green algae. Science. 2002;296:2395–2398. - PubMed
-
- Lórenz-Fonfría VA, Heberle J. Channelrhodopsin unchained: structure and mechanism of a light-gated cation channel. Biochim Biophys Acta. 2014;1837:626–642. - PubMed
-
- Nagel G, et al. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol. 2005;15:2279–2284. - PubMed
-
- Wietek J, Prigge M. In: Enhancing Channelrhodopsins: An Overview. Optogenetics: Methods and Protocols, Methods in Molecular Biology. Kianianmomeni A, editor. Vol 1408. Humana; Totowa, NJ: 2016. pp. 141–165. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

