Monoterpenyl esters in juvenile mountain pine beetle and sex-specific release of the aggregation pheromone trans-verbenol
- PMID: 29555742
- PMCID: PMC5889670
- DOI: 10.1073/pnas.1722380115
Monoterpenyl esters in juvenile mountain pine beetle and sex-specific release of the aggregation pheromone trans-verbenol
Abstract
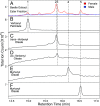
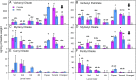
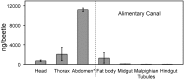
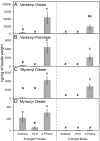
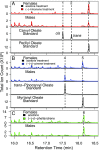
A recent outbreak of mountain pine beetle (MPB) has spread over more than 25 million hectares of pine forests in western North America, affecting pine species of sensitive boreal and mountain ecosystems. During initial host colonization, female MPB produce and release the aggregation pheromone trans-verbenol to coordinate a mass attack of individual trees. trans-Verbenol is formed by hydroxylation of α-pinene, a monoterpene of the pine oleoresin defense. It is thought that adult females produce and immediately release trans-verbenol when encountering α-pinene on a new host tree. Here, we show that both sexes of MPB accumulate the monoterpenyl esters verbenyl oleate and verbenyl palmitate during their development in the brood tree. Verbenyl oleate and verbenyl palmitate were retained in adult female MPB until the time of emergence from brood trees, but were depleted in males. Adult females released trans-verbenol in response to treatment with juvenile hormone III (JHIII). While both sexes produced verbenyl esters when exposed to α-pinene, only females responded to JHIII with release of trans-verbenol. Accumulation of verbenyl esters at earlier life stages may allow adult females to release the aggregation pheromone trans-verbenol upon landing on a new host tree, independent of access to α-pinene. Formation of verbenyl esters may be part of a general detoxification system to overcome host monoterpene defenses in both sexes, from which a specialized and female-specific system of pheromone biosynthesis and release may have evolved.
Keywords: bark beetle; forest health; pheromone; pinene; verbenol.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The cytochrome P450 CYP6DE1 catalyzes the conversion of α-pinene into the mountain pine beetle aggregation pheromone trans-verbenol.Sci Rep. 2019 Feb 6;9(1):1477. doi: 10.1038/s41598-018-38047-8. Sci Rep. 2019. PMID: 30728428 Free PMC article.
-
Pheromone Production by an Invasive Bark Beetle Varies with Monoterpene Composition of its Naïve Host.J Chem Ecol. 2015 Jun;41(6):540-9. doi: 10.1007/s10886-015-0590-x. Epub 2015 May 27. J Chem Ecol. 2015. PMID: 26014128
-
Chemical similarity between historical and novel host plants promotes range and host expansion of the mountain pine beetle in a naïve host ecosystem.New Phytol. 2014 Feb;201(3):940-950. doi: 10.1111/nph.12573. Epub 2013 Oct 30. New Phytol. 2014. PMID: 24400902
-
Mountain Pine Beetle Epidemic: An Interplay of Terpenoids in Host Defense and Insect Pheromones.Annu Rev Plant Biol. 2022 May 20;73:475-494. doi: 10.1146/annurev-arplant-070921-103617. Epub 2022 Feb 7. Annu Rev Plant Biol. 2022. PMID: 35130442 Review.
-
Pheromone production in bark beetles.Insect Biochem Mol Biol. 2010 Oct;40(10):699-712. doi: 10.1016/j.ibmb.2010.07.013. Epub 2010 Aug 19. Insect Biochem Mol Biol. 2010. PMID: 20727970 Review.
Cited by
-
Tolerance to dietary linalool primarily involves co-expression of cytochrome P450s and cuticular proteins in Pagiophloeus tsushimanus (Coleoptera: Curculionidae) larvae using SMRT sequencing and RNA-seq.BMC Genomics. 2023 Jan 19;24(1):34. doi: 10.1186/s12864-023-09117-7. BMC Genomics. 2023. PMID: 36658477 Free PMC article.
-
Divergent Response of Two Bark Beetle-Fungal Symbiotic Systems to Host Monoterpenes Reflects Niche Partitioning Strategies.J Chem Ecol. 2024 Dec;50(12):994-1005. doi: 10.1007/s10886-024-01535-5. Epub 2024 Aug 21. J Chem Ecol. 2024. PMID: 39167252
-
The cytochrome P450 CYP6DE1 catalyzes the conversion of α-pinene into the mountain pine beetle aggregation pheromone trans-verbenol.Sci Rep. 2019 Feb 6;9(1):1477. doi: 10.1038/s41598-018-38047-8. Sci Rep. 2019. PMID: 30728428 Free PMC article.
-
RNA-Seq used to identify ipsdienone reductase (IDONER): A novel monoterpene carbon-carbon double bond reductase central to Ips confusus pheromone production.Insect Biochem Mol Biol. 2021 Feb;129:103513. doi: 10.1016/j.ibmb.2020.103513. Epub 2021 Jan 1. Insect Biochem Mol Biol. 2021. PMID: 33388375 Free PMC article.
-
Naïve Pine Terpene Response to the Mountain Pine Beetle (Dendroctonus ponderosae) through the Seasons.J Chem Ecol. 2023 Jun;49(5-6):299-312. doi: 10.1007/s10886-023-01418-1. Epub 2023 Mar 16. J Chem Ecol. 2023. PMID: 36929332
References
-
- Meddens AJ, Hicke JA, Ferguson CA. Spatiotemporal patterns of observed bark beetle caused mortality in British Columbia and the western United States. Ecol Appl. 2012;22:1876–1891. - PubMed
-
- Borden JH, et al. Response of the mountain pine beetle, Dendroctonus ponderosae Hopkins (Coleoptera: Scolytidae), to five semiochemicals in British Columbia lodgepole pine forests. Can J For Res. 1986;17:118–128.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

