Dimeric sorting code for concentrative cargo selection by the COPII coat
- PMID: 29555761
- PMCID: PMC5889621
- DOI: 10.1073/pnas.1704639115
Dimeric sorting code for concentrative cargo selection by the COPII coat
Abstract
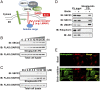
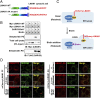
The flow of cargo vesicles along the secretory pathway requires concerted action among various regulators. The COPII complex, assembled by the activated SAR1 GTPases on the surface of the endoplasmic reticulum, orchestrates protein interactions to package cargos and generate transport vesicles en route to the Golgi. The dynamic nature of COPII, however, hinders analysis with conventional biochemical assays. Here we apply proximity-dependent biotinylation labeling to capture the dynamics of COPII transport in cells. When SAR1B was fused with a promiscuous biotin ligase, BirA*, the fusion protein SAR1B-BirA* biotinylates and thus enables the capture of COPII machinery and cargos in a GTP-dependent manner. Biochemical and pulse-chase imaging experiments demonstrate that the COPII coat undergoes a dynamic cycle of engagement-disengagement with the transmembrane cargo receptor LMAN1/ERGIC53. LMAN1 undergoes a process of concentrative sorting by the COPII coat, via a dimeric sorting code generated by oligomerization of the cargo receptor. Similar oligomerization events have been observed with other COPII sorting signals, suggesting that dimeric/multimeric sorting codes may serve as a general mechanism to generate selectivity of cargo sorting.
Keywords: COPII; LMAN1; cargo receptor; cargo sorting; proximity-dependent biotinylation.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
COPII and the regulation of protein sorting in mammals.Nat Cell Biol. 2011 Dec 22;14(1):20-8. doi: 10.1038/ncb2390. Nat Cell Biol. 2011. PMID: 22193160 Review.
-
Mechanisms of COPII vesicle formation and protein sorting.FEBS Lett. 2007 May 22;581(11):2076-82. doi: 10.1016/j.febslet.2007.01.091. Epub 2007 Feb 14. FEBS Lett. 2007. PMID: 17316621 Review.
-
COPII coat assembly and selective export from the endoplasmic reticulum.J Biochem. 2004 Dec;136(6):755-60. doi: 10.1093/jb/mvh184. J Biochem. 2004. PMID: 15671485 Review.
-
Secretory bulk flow of soluble proteins is efficient and COPII dependent.Plant Cell. 2001 Sep;13(9):2005-20. doi: 10.1105/tpc.010110. Plant Cell. 2001. PMID: 11549760 Free PMC article.
-
Making COPII coats.Cell. 2007 Jun 29;129(7):1251-2. doi: 10.1016/j.cell.2007.06.015. Cell. 2007. PMID: 17604713 Review.
Cited by
-
How to Avoid a No-Deal ER Exit.Cells. 2019 Sep 7;8(9):1051. doi: 10.3390/cells8091051. Cells. 2019. PMID: 31500301 Free PMC article. Review.
-
Lysosomal targeting of the ABC transporter TAPL is determined by membrane-localized charged residues.J Biol Chem. 2019 May 3;294(18):7308-7323. doi: 10.1074/jbc.RA118.007071. Epub 2019 Mar 15. J Biol Chem. 2019. PMID: 30877195 Free PMC article.
-
Cholix protein domain I functions as a carrier element for efficient apical to basal epithelial transcytosis.Tissue Barriers. 2020;8(1):1710429. doi: 10.1080/21688370.2019.1710429. Epub 2020 Jan 13. Tissue Barriers. 2020. PMID: 31928299 Free PMC article.
-
Efficient progranulin exit from the ER requires its interaction with prosaposin, a Surf4 cargo.J Cell Biol. 2022 Feb 7;221(2):e202104044. doi: 10.1083/jcb.202104044. Epub 2021 Dec 17. J Cell Biol. 2022. PMID: 34919127 Free PMC article.
-
COPII cage assembly factor Sec13 integrates information flow regulating endomembrane function in response to human variation.Sci Rep. 2024 May 3;14(1):10160. doi: 10.1038/s41598-024-60687-2. Sci Rep. 2024. PMID: 38698045 Free PMC article.
References
-
- Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. - PubMed
-
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975;189:347–358. - PubMed
-
- Barlowe C, Helenius A. Cargo capture and bulk flow in the early secretory pathway. Annu Rev Cell Dev Biol. 2016;32:197–222. - PubMed
-
- Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell. 2007;12:671–682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

