Converting Escherichia coli into an archaebacterium with a hybrid heterochiral membrane
- PMID: 29555770
- PMCID: PMC5889666
- DOI: 10.1073/pnas.1721604115
Converting Escherichia coli into an archaebacterium with a hybrid heterochiral membrane
Abstract
One of the main differences between bacteria and archaea concerns their membrane composition. Whereas bacterial membranes are made up of glycerol-3-phosphate ester lipids, archaeal membranes are composed of glycerol-1-phosphate ether lipids. Here, we report the construction of a stable hybrid heterochiral membrane through lipid engineering of the bacterium Escherichia coli By boosting isoprenoid biosynthesis and heterologous expression of archaeal ether lipid biosynthesis genes, we obtained a viable E. coli strain of which the membranes contain archaeal lipids with the expected stereochemistry. It has been found that the archaeal lipid biosynthesis enzymes are relatively promiscuous with respect to their glycerol phosphate backbone and that E. coli has the unexpected potential to generate glycerol-1-phosphate. The unprecedented level of 20-30% archaeal lipids in a bacterial cell has allowed for analyzing the effect on the mixed-membrane cell's phenotype. Interestingly, growth rates are unchanged, whereas the robustness of cells with a hybrid heterochiral membrane appeared slightly increased. The implications of these findings for evolutionary scenarios are discussed.
Keywords: archaea; bacteria; ether lipids; hybrid membranes; lipid biosynthesis.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


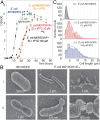

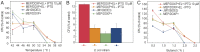
Comment in
-
Engineering E. coli to Have a Hybrid Archaeal/Bacterial Membrane.Trends Microbiol. 2018 Jul;26(7):559-560. doi: 10.1016/j.tim.2018.05.003. Epub 2018 May 19. Trends Microbiol. 2018. PMID: 29789226
-
Engineered bacterium fuels evolution debate.Nature. 2019 Jul;571(7765):326. doi: 10.1038/d41586-019-02163-w. Nature. 2019. PMID: 31312064 No abstract available.
References
-
- Koga Y, Kyuragi T, Nishihara M, Sone N. Did archaeal and bacterial cells arise independently from noncellular precursors? A hypothesis stating that the advent of membrane phospholipid with enantiomeric glycerophosphate backbones caused the separation of the two lines of descent. J Mol Evol. 1998;46:54–63. - PubMed
-
- Maurer SE, Deamer DW, Boncella JM, Monnard PA. Chemical evolution of amphiphiles: glycerol monoacyl derivatives stabilize plausible prebiotic membranes. Astrobiology. 2009;9:979–987. - PubMed
-
- Pozzi G, et al. Single-chain polyprenyl phosphates form “primitive” membranes. Angew Chem Int Ed Engl. 1996;35:177–180.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

