Ribosomal RNA Biogenesis and Its Response to Chilling Stress in Oryza sativa
- PMID: 29555785
- PMCID: PMC5933117
- DOI: 10.1104/pp.17.01714
Ribosomal RNA Biogenesis and Its Response to Chilling Stress in Oryza sativa
Abstract
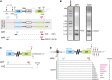
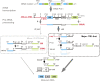
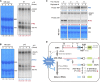
Ribosome biogenesis is crucial for plant growth and environmental acclimation. Processing of ribosomal RNAs (rRNAs) is an essential step in ribosome biogenesis and begins with transcription of the rDNA. The resulting precursor-rRNA (pre-rRNA) transcript undergoes systematic processing, where multiple endonucleolytic and exonucleolytic cleavages remove the external and internal transcribed spacers (ETS and ITS). The processing sites and pathways for pre-rRNA processing have been deciphered in Saccharomyces cerevisiae and, to some extent, in Xenopus laevis, mammalian cells, and Arabidopsis (Arabidopsis thaliana). However, the processing sites and pathways remain largely unknown in crops, particularly in monocots such as rice (Oryza sativa), one of the most important food resources in the world. Here, we identified the rRNA precursors produced during rRNA biogenesis and the critical endonucleolytic cleavage sites in the transcribed spacer regions of pre-rRNAs in rice. We further found that two pre-rRNA processing pathways, distinguished by the order of 5' ETS removal and ITS1 cleavage, coexist in vivo. Moreover, exposing rice to chilling stress resulted in the inhibition of rRNA biogenesis mainly at the pre-rRNA processing level, suggesting that these energy-intensive processes may be reduced to increase acclimation and survival at lower temperatures. Overall, our study identified the pre-rRNA processing pathway in rice and showed that ribosome biogenesis is quickly inhibited by low temperatures, which may shed light on the link between ribosome biogenesis and environmental acclimation in crop plants.
© 2018 American Society of Plant Biologists. All Rights Reserved.
Figures




Similar articles
-
The nucleolar protein NOL12 is required for processing of large ribosomal subunit rRNA precursors in Arabidopsis.BMC Plant Biol. 2023 Nov 3;23(1):538. doi: 10.1186/s12870-023-04561-9. BMC Plant Biol. 2023. PMID: 37919659 Free PMC article.
-
The roles of SSU processome components and surveillance factors in the initial processing of human ribosomal RNA.RNA. 2014 Apr;20(4):540-50. doi: 10.1261/rna.043471.113. Epub 2014 Feb 18. RNA. 2014. PMID: 24550520 Free PMC article.
-
atBRX1-1 and atBRX1-2 are involved in an alternative rRNA processing pathway in Arabidopsis thaliana.RNA. 2015 Mar;21(3):415-25. doi: 10.1261/rna.047563.114. Epub 2015 Jan 20. RNA. 2015. PMID: 25605960 Free PMC article.
-
Feedback regulation of ribosome assembly.Curr Genet. 2018 Apr;64(2):393-404. doi: 10.1007/s00294-017-0764-x. Epub 2017 Oct 11. Curr Genet. 2018. PMID: 29022131 Review.
-
Processing of preribosomal RNA in Saccharomyces cerevisiae.Wiley Interdiscip Rev RNA. 2015 Mar-Apr;6(2):191-209. doi: 10.1002/wrna.1267. Epub 2014 Oct 18. Wiley Interdiscip Rev RNA. 2015. PMID: 25327757 Review.
Cited by
-
The Arabidopsis 2'-O-Ribose-Methylation and Pseudouridylation Landscape of rRNA in Comparison to Human and Yeast.Front Plant Sci. 2021 Jul 26;12:684626. doi: 10.3389/fpls.2021.684626. eCollection 2021. Front Plant Sci. 2021. PMID: 34381476 Free PMC article. Review.
-
Defective Processing of Cytoplasmic and Chloroplast Ribosomal RNA in the Absence of Arabidopsis DXO1.Plant Cell Environ. 2025 Jun;48(6):4227-4244. doi: 10.1111/pce.15425. Epub 2025 Feb 10. Plant Cell Environ. 2025. PMID: 39927756 Free PMC article.
-
Dynamic Dysregulation of Ribosomal Protein Genes in Mouse Brain Stress Models.Stresses. 2024 Dec;4(4):916-922. doi: 10.3390/stresses4040061. Epub 2024 Dec 12. Stresses. 2024. PMID: 40822572 Free PMC article.
-
Genome-wide transcriptome profiling revealed biological macromolecules respond to low temperature stress in Brassica napus L.Front Plant Sci. 2022 Nov 14;13:1050995. doi: 10.3389/fpls.2022.1050995. eCollection 2022. Front Plant Sci. 2022. PMID: 36452101 Free PMC article.
-
The nucleolar protein NOL12 is required for processing of large ribosomal subunit rRNA precursors in Arabidopsis.BMC Plant Biol. 2023 Nov 3;23(1):538. doi: 10.1186/s12870-023-04561-9. BMC Plant Biol. 2023. PMID: 37919659 Free PMC article.
References
-
- Abbasi N, Kim HB, Park NI, Kim HS, Kim YK, Park YI, Choi SB (2010) APUM23, a nucleolar Puf domain protein, is involved in pre-ribosomal RNA processing and normal growth patterning in Arabidopsis. Plant J 64: 960–976 - PubMed
-
- Al Refaii A, Alix JH (2009) Ribosome biogenesis is temperature-dependent and delayed in Escherichia coli lacking the chaperones DnaK or DnaJ. Mol Microbiol 71: 748–762 - PubMed
-
- Anger AM, Armache JP, Berninghausen O, Habeck M, Subklewe M, Wilson DN, Beckmann R (2013) Structures of the human and Drosophila 80S ribosome. Nature 497: 80–85 - PubMed
-
- Bailey-Serres J, Sorenson R, Juntawong P (2009) Getting the message across: cytoplasmic ribonucleoprotein complexes. Trends Plant Sci 14: 443–453 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

