Betaine Lipid Is Crucial for Adapting to Low Temperature and Phosphate Deficiency in Nannochloropsis
- PMID: 29555786
- PMCID: PMC5933114
- DOI: 10.1104/pp.17.01573
Betaine Lipid Is Crucial for Adapting to Low Temperature and Phosphate Deficiency in Nannochloropsis
Abstract
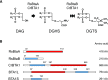
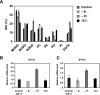
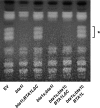
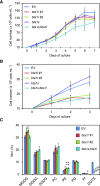
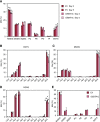
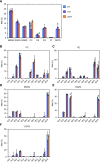
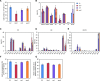
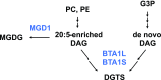
Diacylglyceryl-N,N,N-trimethylhomo-Ser (DGTS) is a nonphosphorous, polar glycerolipid that is regarded as analogous to the phosphatidylcholine in bacteria, fungi, algae, and basal land plants. In some species of algae, including the stramenopile microalga Nannochloropsis oceanica, DGTS contains an abundance of eicosapentaenoic acid (EPA), which is relatively scarce in phosphatidylcholine, implying that DGTS has a unique physiological role. In this study, we addressed the role of DGTS in N. oceanica We identified two DGTS biosynthetic enzymes that have distinct domain configurations compared to previously identified DGTS synthases. Mutants lacking DGTS showed growth retardation under phosphate starvation, demonstrating a pivotal role for DGTS in the adaptation to this condition. Under normal conditions, DGTS deficiency led to an increase in the relative amount of monogalactosyldiacylglycerol, a major plastid membrane lipid with high EPA content, whereas excessive production of DGTS induced by gene overexpression led to a decrease in monogalactosyldiacylglycerol. Meanwhile, lipid analysis of partial phospholipid-deficient mutants revealed a role for phosphatidylcholine and phosphatidylethanolamine in EPA biosynthesis. These results suggest that DGTS and monogalactosyldiacylglycerol may constitute the two major pools of EPA in extraplastidic and plastidic membranes, partially competing to acquire EPA or its precursors derived from phospholipids. The mutant lacking DGTS also displayed impaired growth and a lower proportion of EPA in extraplastidic compartments at low temperatures. Our results indicate that DGTS is involved in the adaptation to low temperatures through a mechanism that is distinct from the DGTS-dependent adaptation to phosphate starvation in N. oceanica.
© 2018 American Society of Plant Biologists. All Rights Reserved.
Figures
Similar articles
-
Do betaine lipids replace phosphatidylcholine as fatty acid editing hubs in microalgae?Front Plant Sci. 2023 Jan 19;14:1077347. doi: 10.3389/fpls.2023.1077347. eCollection 2023. Front Plant Sci. 2023. PMID: 36743481 Free PMC article. Review.
-
Phosphate starvation in fungi induces the replacement of phosphatidylcholine with the phosphorus-free betaine lipid diacylglyceryl-N,N,N-trimethylhomoserine.Eukaryot Cell. 2014 Jun;13(6):749-57. doi: 10.1128/EC.00004-14. Epub 2014 Apr 11. Eukaryot Cell. 2014. PMID: 24728191 Free PMC article.
-
The effect of phosphate starvation on the lipid and fatty acid composition of the fresh water eustigmatophyte Monodus subterraneus.Phytochemistry. 2006 Apr;67(7):696-701. doi: 10.1016/j.phytochem.2006.01.010. Epub 2006 Feb 23. Phytochemistry. 2006. PMID: 16497342
-
A Palmitic Acid Elongase Affects Eicosapentaenoic Acid and Plastidial Monogalactosyldiacylglycerol Levels in Nannochloropsis.Plant Physiol. 2017 Jan;173(1):742-759. doi: 10.1104/pp.16.01420. Epub 2016 Nov 28. Plant Physiol. 2017. PMID: 27895203 Free PMC article.
-
Betaine lipids: Biosynthesis, functional diversity and evolutionary perspectives.Prog Lipid Res. 2025 Jan;97:101320. doi: 10.1016/j.plipres.2025.101320. Epub 2025 Jan 8. Prog Lipid Res. 2025. PMID: 39793901 Review.
Cited by
-
Do betaine lipids replace phosphatidylcholine as fatty acid editing hubs in microalgae?Front Plant Sci. 2023 Jan 19;14:1077347. doi: 10.3389/fpls.2023.1077347. eCollection 2023. Front Plant Sci. 2023. PMID: 36743481 Free PMC article. Review.
-
Suboptimal Temperature Acclimation Affects Kennedy Pathway Gene Expression, Lipidome and Metabolite Profile of Nannochloropsis salina during PUFA Enriched TAG Synthesis.Mar Drugs. 2018 Nov 1;16(11):425. doi: 10.3390/md16110425. Mar Drugs. 2018. PMID: 30388843 Free PMC article.
-
Site-Specific Lipidomic Signatures of Sea Lettuce (Ulva spp., Chlorophyta) Hold the Potential to Trace Their Geographic Origin.Biomolecules. 2020 Mar 23;10(3):489. doi: 10.3390/biom10030489. Biomolecules. 2020. PMID: 32210093 Free PMC article.
-
Development of a high-performance thin-layer chromatography-based method for targeted glycerolipidome profiling of microalgae.Anal Bioanal Chem. 2024 Feb;416(5):1149-1164. doi: 10.1007/s00216-023-05101-y. Epub 2024 Jan 4. Anal Bioanal Chem. 2024. PMID: 38172195 Free PMC article.
-
Lipidomics Analysis of Multilamellar Bodies Produced by Amoeba Acanthamoeba castellanii in Co-Culture with Klebsiella aerogenes.Pathogens. 2023 Mar 3;12(3):411. doi: 10.3390/pathogens12030411. Pathogens. 2023. PMID: 36986333 Free PMC article.
References
-
- Babiychuk E, Müller F, Eubel H, Braun HP, Frentzen M, Kushnir S (2003) Arabidopsis phosphatidylglycerophosphate synthase 1 is essential for chloroplast differentiation, but is dispensable for mitochondrial function. Plant J 33: 899–909 - PubMed
-
- Benning C, Huang ZH, Gage DA (1995) Accumulation of a novel glycolipid and a betaine lipid in cells of Rhodobacter sphaeroides grown under phosphate limitation. Arch Biochem Biophys 317: 103–111 - PubMed
-
- Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

