Protein Storage Vacuoles Originate from Remodeled Preexisting Vacuoles in Arabidopsis thaliana
- PMID: 29555788
- PMCID: PMC5933143
- DOI: 10.1104/pp.18.00010
Protein Storage Vacuoles Originate from Remodeled Preexisting Vacuoles in Arabidopsis thaliana
Abstract
Protein storage vacuoles (PSV) are the main repository of protein in dicotyledonous seeds, but little is known about the origins of these transient organelles. PSV are hypothesized to either arise de novo or originate from the preexisting embryonic vacuole (EV) during seed maturation. Here, we tested these hypotheses by studying PSV formation in Arabidopsis (Arabidopsis thaliana) embryos at different stages of seed maturation and recapitulated this process in Arabidopsis leaves reprogrammed to an embryogenic fate by inducing expression of the LEAFY COTYLEDON2 transcription factor. Confocal and immunoelectron microscopy indicated that both storage proteins and tonoplast proteins typical of PSV were delivered to the preexisting EV in embryos or to the lytic vacuole in reprogrammed leaf cells. In addition, sectioning through embryos at several developmental stages using serial block face scanning electron microscopy revealed the 3D architecture of forming PSV. Our results indicate that the preexisting EV is reprogrammed to become a PSV in Arabidopsis.
© 2018 American Society of Plant Biologists. All Rights Reserved.
Figures


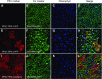
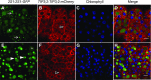



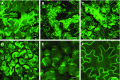

Comment in
-
Turnover of Tonoplast Proteins.Plant Physiol. 2018 May;177(1):10-11. doi: 10.1104/pp.18.00322. Plant Physiol. 2018. PMID: 29720533 Free PMC article. No abstract available.
References
-
- Bagga S, Sutton D, Kemp JD, Sengupta-Gopalan C (1992) Constitutive expression of the β-phaseolin gene in different tissues of transgenic alfalfa does not ensure phaseolin accumulation in non-seed tissue. Plant Mol Biol 19: 951–958 - PubMed
-
- Baud S, Kelemen Z, Thévenin J, Boulard C, Blanchet S, To A, Payre M, Berger N, Effroy-Cuzzi D, Franco-Zorrilla JM, et al. (2016) Deciphering the molecular mechanisms underpinning the transcriptional control of gene expression by master transcriptional regulators in Arabidopsis seed. Plant Physiol 171: 1099–1112 - PMC - PubMed
-
- Bolte S, Lanquar V, Soler MN, Beebo A, Satiat-Jeunemaître B, Bouhidel K, Thomine S (2011) Distinct lytic vacuolar compartments are embedded inside the protein storage vacuole of dry and germinating Arabidopsis thaliana seeds. Plant Cell Physiol 52: 1142–1152 - PubMed
-
- Bolte S, Talbot C, Boutte Y, Catrice O, Read ND, Satiat-Jeunemaitre B (2004) FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells. J Microsc 214: 159–173 - PubMed
-
- Braybrook SA, Harada JJ (2008) LECs go crazy in embryo development. Trends Plant Sci 13: 624–630 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

