The structure of hydrogenase-2 from Escherichia coli: implications for H2-driven proton pumping
- PMID: 29555844
- PMCID: PMC5902676
- DOI: 10.1042/BCJ20180053
The structure of hydrogenase-2 from Escherichia coli: implications for H2-driven proton pumping
Abstract
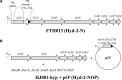
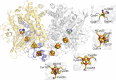
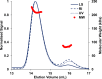
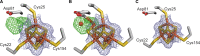
Under anaerobic conditions, Escherichia coli is able to metabolize molecular hydrogen via the action of several [NiFe]-hydrogenase enzymes. Hydrogenase-2, which is typically present in cells at low levels during anaerobic respiration, is a periplasmic-facing membrane-bound complex that functions as a proton pump to convert energy from hydrogen (H2) oxidation into a proton gradient; consequently, its structure is of great interest. Empirically, the complex consists of a tightly bound core catalytic module, comprising large (HybC) and small (HybO) subunits, which is attached to an Fe-S protein (HybA) and an integral membrane protein (HybB). To date, efforts to gain a more detailed picture have been thwarted by low native expression levels of Hydrogenase-2 and the labile interaction between HybOC and HybA/HybB subunits. In the present paper, we describe a new overexpression system that has facilitated the determination of high-resolution crystal structures of HybOC and, hence, a prediction of the quaternary structure of the HybOCAB complex.
Keywords: NiFe hydrogenase; crystallography; electrochemistry; hydrogen metabolism; iron–sulfur clusters; metalloenzymes.
© 2018 The Author(s).
Conflict of interest statement
The Authors declare that there are no competing interests associated with the manuscript.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

