Scoulerine affects microtubule structure, inhibits proliferation, arrests cell cycle and thus culminates in the apoptotic death of cancer cells
- PMID: 29555944
- PMCID: PMC5859271
- DOI: 10.1038/s41598-018-22862-0
Scoulerine affects microtubule structure, inhibits proliferation, arrests cell cycle and thus culminates in the apoptotic death of cancer cells
Abstract
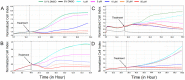
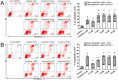
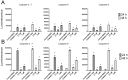
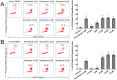
Scoulerine is an isoquinoline alkaloid, which indicated promising suppression of cancer cells growth. However, the mode of action (MOA) remained unclear. Cytotoxic and antiproliferative properties were determined in this study. Scoulerine reduces the mitochondrial dehydrogenases activity of the evaluated leukemic cells with IC50 values ranging from 2.7 to 6.5 µM. The xCELLigence system revealed that scoulerine exerted potent antiproliferative activity in lung, ovarian and breast carcinoma cell lines. Jurkat and MOLT-4 leukemic cells treated with scoulerine were decreased in proliferation and viability. Scoulerine acted to inhibit proliferation through inducing G2 or M-phase cell cycle arrest, which correlates well with the observed breakdown of the microtubule network, increased Chk1 Ser345, Chk2 Thr68 and mitotic H3 Ser10 phosphorylation. Scoulerine was able to activate apoptosis, as determined by p53 upregulation, increase caspase activity, Annexin V and TUNEL labeling. Results highlight the potent antiproliferative and proapoptotic function of scoulerine in cancer cells caused by its ability to interfere with the microtubule elements of the cytoskeleton, checkpoint kinase signaling and p53 proteins. This is the first study of the mechanism of scoulerine at cellular and molecular level. Scoulerine is a potent antimitotic compound and that it merits further investigation as an anticancer drug.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Chlebek J, et al. Acetylcholinesterase and butyrylcholinesterase inhibitory compounds from Corydalis cava (Fumariaceae) Natural Product Communications. 2011;6:607–610. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

