Nucleotide-mediated SPDEF modulates TFF3-mediated wound healing and intestinal barrier function during the weaning process
- PMID: 29555969
- PMCID: PMC5859294
- DOI: 10.1038/s41598-018-23218-4
Nucleotide-mediated SPDEF modulates TFF3-mediated wound healing and intestinal barrier function during the weaning process
Abstract
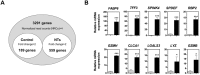
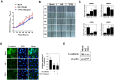
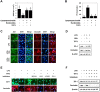
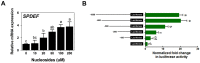
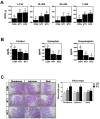
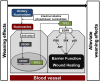
Most alterations during weaning involve physiological changes in intestinal structure and function. Here, we evaluated the molecular mechanisms regulating the effects of nucleotides on weaning. Nucleotide treatment induced Trefoil factor 3 (TFF3) expression and IPEC-J2 cell growth and reduced wound width. Treatment with nucleosides and TFF3 in lipopolysaccharide-challenged IPEC-J2 cells increased intestinal transepithelial electrical resistance and decreased intestinal permeability. Additionally, nucleosides improved intestinal barrier function through induction of TFF3-mediated phosphatidylinositol 3-kinase/Akt, extracellular signal-regulated kinase 1/2, p38, and Janus kinase/signal transducer and activator of transcription signaling pathways. Among selected differentially expressed genes, SAM pointed domain containing ETS transcription factor (SPDEF) expression was elevated by nucleotides in a concentration-dependent manner. Moreover, SPDEF directly regulated TFF3 expression via binding to the promoter. In vivo, nucleotide supplementation improved growth performance, serum stress levels, and intestinal morphology. Our findings provide insights into the molecular mechanisms of intestinal development during weaning in pigs.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

