Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex
- PMID: 29556025
- PMCID: PMC5920726
- DOI: 10.1038/s41593-018-0121-5
Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex
Abstract
Oligodendrocyte generation in the adult CNS provides a means to adapt the properties of circuits to changes in life experience. However, little is known about the dynamics of oligodendrocytes and the extent of myelin remodeling in the mature brain. Using longitudinal in vivo two-photon imaging of oligodendrocytes and their progenitors in the mouse cerebral cortex, we show that myelination is an inefficient and extended process, with half of the final complement of oligodendrocytes generated after 4 months of age. Oligodendrocytes that successfully integrated formed new sheaths on unmyelinated and sparsely myelinated axons, and they were extremely stable, gradually changing the pattern of myelination. Sensory enrichment robustly increased oligodendrocyte integration, but did not change the length of existing sheaths. This experience-dependent enhancement of myelination in the mature cortex may accelerate information transfer in these circuits and strengthen the ability of axons to sustain activity by providing additional metabolic support.
Conflict of interest statement
Figures

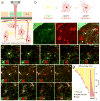



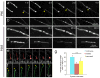


Comment in
-
Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain.Nat Neurosci. 2018 May;21(5):683-695. doi: 10.1038/s41593-018-0120-6. Epub 2018 Mar 19. Nat Neurosci. 2018. PMID: 29556031 Free PMC article.
References
-
- Nave KA. Myelination and support of axonal integrity by glia. Nature. 2010;468:244–252. - PubMed
-
- Yakovlev P, Lecours A. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional development of the brain in early life. Vol. 1967 Oxford: Blackwell Scientific Publications; 1967.
-
- Baumann N, Pham-Dinh D. Biology of Oligodendrocyte and Myelin in the Mammalian Central Nervous System. Physiol Rev. 2001;81:871–927. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

